Abstract
Purpose
The effect of monotherapy in cancer is frequently influenced by the tumor’s unique hypoxic microenvironment, insufficient drug concentration at the treatment site, and tumour cells’ increased drug tolerance. In this work, we expect to design a novel therapeutic nanoprobe with the ability to solve these problems and improve the efficacy of antitumor therapy.
Methods
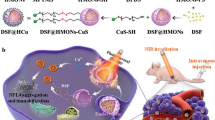
We have prepared a hollow manganese dioxide nanoprobes loaded with photosensitive drug IR780 for the photothermal/photodynamic/chemodynamic co-therapy of liver cancer.
Results
The nanoprobe demonstrates efficient thermal transformation ability under a single laser irradiation, and under the synergistic influence of photo heat, accelerates the Fenton/ Fenton-like reaction efficiency based on Mn2+ ions to produce more ·OH under the synergistic effect of photo heat. Moreover, the oxygen released under the degradation of manganese dioxide further promotes the ability of photosensitive drugs to produce singlet oxygen (ROS). The nanoprobe has been found to efficiently destroy tumour cells in vivo and in vitro experiments when used in combination with photothermal/photodynamic/ chemodynamic modes of treatment under laser irradiation.
Conclusion
In all, this research shows that a therapeutic strategy based on this nanoprobe could be a viable alternative for cancer treatment in the near future.








Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Condron CM. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29(4):297–307.
Michael; Dean; Tito; Fojo; Susan; Bates. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84.
Antaris AL, Robinson JT, Yaghi OK, Hong G, Diao S, Luong R, Dai H. Ultra-low doses of chirality sorted (6,5) carbon nanotubes for simultaneous tumor imaging and photothermal therapy. ACS Nano. 2013;7(4):3644–52.
Xu XL, Chen MX, Lou XF, Du YY, Du YZ. Sialic acid-modified mesoporous polydopamine induces tumor vessel normalization to enhance photodynamic therapy by inhibiting VE-cadherin internalization. Chem Eng J. 2021;414(1): 128743.
Li Z, Fan J, Tong C, Zhou H, Wang W. A smart drug-delivery nanosystem based on carboxylated graphene quantum dots for tumor-targeted chemotherapy. Nanomedicine. 2019;14(15):2011–25.
Feng L, Gai S, He F, Yang P, Zhao Y. Multifunctional Bismuth Ferrite Nanocatalysts with Optical and Magnetic Functions for Ultrasound Enhanced Tumor Theranostics. ACS Nano. 2020;14(6):7245–58.
Zhang Y, Miao Q, Pu K. Activatable Polymeric Nanoprobe for Near-Infrared Fluorescence and Photoacoustic Imaging of T Lymphocytes. Angew Chem. 2020;133(11):5986–92.
Yao D, Wang Y, Zou R, Bian K, Wang D. Molecular Engineered Squaraine Nanoprobe for NIR-II/Photoacoustic Imaging and Photothermal Therapy of Metastatic Breast Cancer. ACS Appl Mater Interfaces. 2020;12(4):4276–84.
Zhang, Tinghui, Yin, Li, Rongqin, Zheng, Chen, Qiu, Kit, S, Lam. Size Modulable Nanoprobe for High-Performance Ultrasound Imaging and Drug Delivery Against Cancer. ACS Nano. 2018;12(4):3449–60.
Haoyu Wang, Yan-YanFu, Xuejun Zhang, ChunshuiYu, Shao-KaiSun. Hyaluronic acid-mediated one-pot facile synthesis of a sensitive and biocompatible Gd2 O3 nanoprobe for MR imaging in vivo. RSC Adv. 2015;5(113):93041–7.
Bouché M, Pühringer M, Iturmendi A, Amirshaghaghi A, Cormode DP. Activatable Hybrid Polyphosphazene-AuNP Nanoprobe for ROS Detection by Bimodal PA/CT Imaging. ACS Appl Mater Interfaces. 2019;11(32):28648–56.
Chen Y, Cong H, Shen Y, Yu B. Biomedical Application of Manganese Dioxide Nanomaterials. Nanotechnology. 2020;31(20): 202001.
Liu X, Zhou Y, Xie W, Liu S, Zhao Q, Huang W. Construction of Smart Manganese Dioxide-Based All-in-One Nanoplatform for Cancer Diagnosis and Therapy. Small Methods. 2020;4:2000566.
Fan S, Zhang Y, Tan H, Xue C, Cui D. Manganese/Iron-Based Nanoprobes for Photodynamic/Chemotherapy Combination Therapy of Tumor Guided by Multimodal Imaging. Nanoscale. 2021;13(10):5383–99.
Xu KF, Jia HR, Zhu YX, Liu X, Wu FG. Cholesterol-Modified Dendrimers for Constructing a Tumor Microenvironment-Responsive Drug Delivery System. ACS Biomater Sci Eng. 2019;5(11):6072–81.
Zhu W, Dong Z, Fu T, Liu J, Chen Q, Li Y, Zhu R, Xu L, Liu Z. Modulation of Hypoxia in Solid Tumor Microenvironment with MnO2 Nanoparticles to Enhance Photodynamic Therapy. Adv Func Mater. 2016;26(30):5490–8.
Wang H, Bremner DH, Wu K, Gong X, Zhu LM. Platelet Membrane Biomimetic Bufalin-Loaded Hollow MnO2 Nanoparticles for MRI-Guided Chemo-Chemodynamic Combined Therapy of Cancer. Chem Eng J. 2019;382: 122848.
Xu X, Duan J, Liu Y, Kuang Y, Li C. Multi-stimuli responsive hollow MnO2-based drug delivery system for magnetic resonance imaging and combined chemo-chemodynamic cancer therapy. Acta Biomater. 2021;126:445–62.
Manivasagan P, Joe A, Han HW, Thambi T, Jang ES. Recent advances in multifunctional nanomaterials for photothermal-enhanced Fenton-based chemodynamic tumor therapy. Materials Today Bio. 2022;13(7494): 100197.
Cheng Y, Lu H, Yang F, Zhang Y, Dong H. Biodegradable FeWOx Nanoparticles for CT/MR Imaging-Guided Synergistic Photothermal. Photodynamic and Chemodynamic Therapy Nanoscale. 2021;13(5):3049–60.
Geng B, Zhang S, Yang X, Shi W, Li P, Pan D, Shen L. Cu2-xO@TiO2-y Z-scheme heterojunctions for sonodynamic-chemodynamic combined tumor eradication. Chem Eng J. 2022;435: 134777.
Chen Q, Ma Y, Bai P, Li Q, Li C. Tumor Microenvironment-Responsive Nanococktails for Synergistic Enhancement of Cancer Treatment via Cascade Reactions. ACS Appl Mater Interfaces. 2021;13(4):4861–73.
Wang Y, Shi L, Ye Z, Guan K, Zhang XB. Reactive Oxygen Correlated Chemiluminescent Imaging of Semiconducting Polymer Nanoplatform for Monitoring Chemodynamic Therapy. Nano Lett. 2019;20(1):176–83.
Liu Y, Li H, Li S, Zhang X, Xiong J, Jiang F, Liu Y, Jiang P. Chiral Cu 2–x Se Nanoparticles for Enhanced Synergistic Cancer Chemodynamic/Photothermal Therapy in the Second Near-Infrared Biowindow. ACS Appl Mater Interfaces. 2021;13(51):60933–44.
Ma B, Nishina Y, Bianco A. A glutathione responsive nanoplatform made of reduced graphene oxide and MnO2 nanoparticles for photothermal and chemodynamic combined therapy. Carbon. 2021;178(4):783–91.
Han X, Xu Y, Li Y, Zhao X, Nie G. An Extendable Star-Like Nanoplatform for Functional and Anatomical Imaging-Guided Photothermal Oncotherapy. ACS Nano. 2019;13(4):4379–91.
Wang Y, Meng HM, Song G, Li Z, Zhang XB. Conjugated-Polymer-Based Nanomaterials for Photothermal Therapy. ACS Applied Polymer Materials. 2020;2(10):4258–72.
Li Z, Huang P, Zhang X, Lin J, Yang S, Liu B, Gao F, Xi P, Ren Q, Cui D. RGD-Conjugated Dendrimer-Modified Gold Nanorods for in Vivo Tumor Targeting and Photothermal Therapy. Mol Pharm. 2010;7(1):94–104.
Yuan Z, Lin C, He Y, Tao B, Chen M, Zhang J, Liu P, Cai K. Near-Infrared Light-Triggered Nitric-Oxide-Enhanced Photodynamic Therapy and Low-Temperature Photothermal Therapy for Biofilm Elimination. ACS Nano. 2020;24(3):3546–62.
Yu Yang, Wenjun Zhu, Ziliang Dong, Yu Chao, Lai Xu. Photothermal Therapy: 1D Coordination Polymer Nanofibers for Low-Temperature Photothermal Therapy (Adv. Mater. 40/2017). Adv Mater. 2017;29(40):1703588.
Qian C, Liang C, Wang C, Liu Z. An Imagable and Photothermal “Abraxane-Like” Nanodrug for Combination Cancer Therapy to Treat Subcutaneous and Metastatic Breast Tumors. Adv Mater. 2015;27(5):903–10.
Acknowledgements
This study was supported by Department of Science and Technology of Sichuan Province (No. 2021YFSY0038 and 2022NSFSC0680).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, B., Ye, X., Fu, Y. et al. Hollow MnO2-Based Nanoprobes for Enhanced Photothermal/Photodynamic /Chemodynamic Co-Therapy of Hepatocellular Carcinoma. Pharm Res 40, 1271–1282 (2023). https://doi.org/10.1007/s11095-023-03501-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03501-4