Abstract
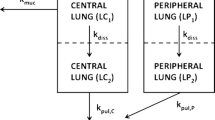
This study aimed to gain an in-depth understanding of the pulmonary fate of three experimental fluticasone propionate (FP) dry powder inhaler formulations which differed in mass median aerodynamic diameters (MMAD; A-4.5 µm, B-3.8 µm and C-3.7 µm; total single dose: 500 µg). Systemic disposition parameter estimates were obtained from published pharmacokinetic data after intravenous dosing to improve robustness. A biphasic pulmonary absorption model, with mucociliary clearance from the slower absorption compartment, and three systemic disposition compartments was most suitable. Rapid absorption, presumably from peripheral lung, had half-lives of 6.9 to 14.6 min. The peripherally deposited dose (12.6 µg) was significantly smaller for formulation A-4.5 µm than for the other formulations (38.7 and 39.3 µg for B-3.8 µm and C-3.7 µm). The slow absorption half-lives ranged from 6.86 to 9.13 h and were presumably associated with more central lung regions, where mucociliary clearance removed approximately half of the centrally deposited dose. Simulation-estimation studies showed that a biphasic absorption model could be reliably identified and that parameter estimates were unbiased and reasonably precise. Bioequivalence assessment of population pharmacokinetics derived central and peripheral lung doses suggested that formulation A-4.5 µm lacked bioequivalence compared to the other formulations both for central and peripheral doses. In contrast, the other fomulations were bioequivalent. Overall, population pharmacokinetics holds promise to provide important insights into the pulmonary fate of inhalation drugs, which are not available from non-compartmental analysis. This supports the assessment of the pulmonary bioequivalence of fluticasone propionate inhaled formulations through pharmacokinetic approaches, and may be helpful for discussions on evaluating alternatives to clinical endpoint studies.



Similar content being viewed by others
Data Availability
The raw data for the human PK study were published in AAPS J 2021; 23:48 supported by the US Food and Drug Administration through contracts HHSF223201110117A and HHSF223201610099C and grants 1U01FD004950 and 1U01FD005231. The present population PK analysis did not generate additional experimental data. The population PK modeling andMonte Carlo simulation code is provided in the supplementary materials of the present paper.
References
Hochhaus G, Davis-Cutting C, Oliver M, Lee SL, Lyapustina S. Current Scientific and Regulatory Approaches for Development of Orally Inhaled and Nasal Drug Products: Overview of the IPAC-RS/University of Florida Orlando Inhalation Conference. AAPS J. 2015;17:1305–11.
Lee SL. US regulatory considerations for generic dry powder inhalers. Respiratory drug delivery 2012. Volume 1. In: Dalby RN, Byron PR, Peart J, Suman JD, Young PM, Traini D, editors. River Grove: DHI Publishing; 2012. p. 317–24.
O’Connor D, Adams WP, Chen M-L, Daley-Yates P, Davis J, Derendorf H, et al. Role of pharmacokinetics in establishing bioequivalence for orally inhaled drug products: Workshop summary report. J Aerosol Med Pulm Drug Deliv. 2011;24:119–35.
Adams WP, Ahrens RC, Chen M-L, Christopher D, Chowdhury BA, Conner DP, et al. Demonstrating Bioequivalence of Locally Acting Orally Inhaled Drug Products (OIPs): Workshop Summary Report. J Aerosol Med Pulm Drug Deliv [Internet]. 2010;23:1–29. https://doi.org/10.1089/jamp.2009.0803.
FYs 2013–2017 Regulatory Science Report: Locally-Acting Orally Inhaled and Nasal Drug Products [Internet]. Available from: https://www.fda.gov/drugs/generic-drugs/fys-2013-2017-regulatory-science-report-locally-acting-orally-inhaled-and-nasal-drug-products. Accessed 23 Mar 2023.
Weber B, Hochhaus G. A Systematic Analysis of the Sensitivity of Plasma Pharmacokinetics to Detect Differences in the Pulmonary Performance of Inhaled Fluticasone Propionate Products Using a Model-Based Simulation Approach. AAPS Journal. 2015;17:999–1010 (Springer New York LLC).
Hochhaus G, Chen M-J, Kurumaddali A, Schilling U, Jiao Y, Drescher SK, et al. Can Pharmacokinetic Studies Assess the Pulmonary Fate of Dry Powder Inhaler Formulations of Fluticasone Propionate? AAPS J [Internet]. 2021;23:48. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=33768368&retmode=ref&cmd=prlinks. Accessed 23 Mar 2023.
Thorsson L, Edsbäcker S, Källén A, Löfdahl CG. Pharmacokinetics and systemic activity of fluticasone via Diskus and pMDI, and of budesonide via Turbuhaler. Br J Clin Pharmacol. 2001;52:529–38.
Allen A, Bareille PJ, Rousell VM. Fluticasone furoate, a novel inhaled corticosteroid, demonstrates prolonged lung absorption kinetics in man compared with inhaled fluticasone propionate. Clin Pharmacokinet. 2013;52:37–42.
Hofmann W, Asgharian B. The effect of lung structure on mucociliary clearance and particle retention in human and rat lungs. Toxicol Sci [Internet]. 2003;73:448–56. https://doi.org/10.1093/toxsci/kfg075.
Falcoz C, Oliver R, Mcdowall JE, Ventresca P, Bye A, Daley-Yates PT. Bioavailability of orally administered micronised fluticasone propionate. Clin Pharmacokinet. 2000;39(Suppl. 1):31–9.
Derendorf H, Hochhaus G, Meibohm B, Möllmann H, Barth J. Pharmacokinetics and pharmacodynamics of inhaled corticosteroids. J Allergy Clin Immunol. 1998;101(Supplement):S440–6.
Bauer RJ, Guzy S, Ng C. A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. AAPS J [Internet]. AAPS J; 2007 [cited 2022 Aug 17];9. Available from: https://pubmed.ncbi.nlm.nih.gov/17408237/. Accessed 23 Mar 2023.
Bulitta JB, Bingölbali A, Shin BS, Landersdorfer CB. Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J. 2011;13:201–11.
Bulitta JB, Landersdorfer CB. Performance and robustness of the Monte Carlo importance sampling algorithm using parallelized S-ADAPT for basic and complex mechanistic models. AAPS J [Internet]. AAPS J; 2011 [cited 2022 Aug 17];13:212–26. Available from: https://pubmed.ncbi.nlm.nih.gov/21374103/. Accessed 23 Mar 2023.
Bulitta JB, Okusanya OO, Forrest A, Bhavnani SM, Clark K, Still JG, et al. Population pharmacokinetics of fusidic acid: Rationale for front-loaded dosing regimens due to autoinhibition of clearance. Antimicrob Agents Chemother. 2013;57:498–507.
Brendel K, Comets E, Laffont C, Laveille C, Mentré F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res [Internet]. Springer; 2006 [cited 2022 Aug 17];23:2036–49. Available from: https://doi.org/10.1007/s11095-006-9067-5
Parke J, Holford NHG, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed [Internet]. Comput Methods Programs Biomed; 1999 [cited 2022 Aug 17];59:19–29. Available from: https://pubmed.ncbi.nlm.nih.gov/10215174/. Accessed 23 Mar 2023.
Jiao Y, Kim TH, Tao X, Kinzig M, Landersdorfer CB, Drescher SK, et al. First population pharmacokinetic analysis showing increased quinolone metabolite formation and clearance in patients with cystic fibrosis compared to healthy volunteers. Eur J Pharm Sci. 2018,123:416–28.
Bulitta JB, Duffull SB, Kinzig-Schippers M, Holzgrabe U, Stephan U, Drusano GL, et al. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother [Internet]. Antimicrob Agents Chemother; 2007 [cited 2022 Aug 17];51:2497–507. Available from: https://pubmed.ncbi.nlm.nih.gov/17485505/. Accessed 23 Mar 2023.
Jiao Y, Kim TH, Tao X, Kinzig M, Landersdorfer CB, Drescher SK, et al. First population pharmacokinetic analysis showing increased quinolone metabolite formation and clearance in patients with cystic fibrosis compared to healthy volunteers. Eur J Pharma Sci. 2018;123:416–28 (Elsevier B.V.).
Weber B, Borghardt JM, Parra-Guillen ZP, Sharma A, Retlich S, Staab A, et al. Tiotropium pharmacokinetics and pharmacodynamics – What are drivers for systemic levels and local pulmonary responses? Respiratory Drug Delivery 2016. Volume 1. In: Dalby RN, Byron PR, Peart J, Suman JD, Young PM, Traini D, editors. River Grove: DHI Publishing; 2016. p. 45–54.
Borghardt JM, Weber B, Staab A, Kunz C, Formella S, Kloft C. Investigating pulmonary and systemic pharmacokinetics of inhaled olodaterol in healthy volunteers using a population pharmacokinetic approach. Br J Clin Pharmacol. 2016;81:538–52 (Blackwell Publishing Ltd).
Bartels C, Looby M, Sechaud R, Kaiser G. Determination of the pharmacokinetics of glycopyrronium in the lung using a population pharmacokinetic modelling approach. Br J Clin Pharmacol. 2013;76:868–79.
Brindley C, Falcoz C, Mackie AE, Bye A. Absorption Kinetics after Inhalation of Fluticasone Propionate via the Diskhaler ® , Diskus ® and Metered-Dose Inhaler in Healthy Volunteers. Clin Pharmacokinet. 2000;39(Suppl. 1):1–8.
Soulele K, Macheras P, Silvestro L, RizeaSavu S, Karalis V. Population pharmacokinetics of fluticasone propionate/salmeterol using two different dry powder inhalers. European Journal of Pharmaceutical Sciences Elsevier. 2015;80:33–42.
Boger E, Evans N, Chappell M, Lundqvist A, Ewing P, Wigenborg A, et al. Systems Pharmacology Approach for Prediction of Pulmonary and Systemic Pharmacokinetics and Receptor Occupancy of Inhaled Drugs. CPT Pharmacometrics Syst Pharmacol [Internet]. 2016;5:201–10. https://doi.org/10.1002/psp4.12074.
Biddiscombe MF, Meah SN, Underwood SR, Usmani OS. Comparing lung regions of interest in gamma scintigraphy for assessing inhaled therapeutic aerosol deposition. J Aerosol Med Pulm Drug Deliv [Internet]. 2011;24:165–73. https://doi.org/10.1089/jamp.2010.0845.
Asgharian B, Hofmann W, Bergmann R. Particle deposition in a multiple-path model of the human lung. Aerosol Sci Technol [Internet]. 2001;34:332–9.
Acknowledgements
We thank Drs. Denise Conti, Oluwamurewa (Murewa) Oguntimein, Renishkumar Delvadia, and other FDA colleagues for many fruitful discussions and their many contributions while executing the PK, bioanalytical, and NCA parts of this project. This work has been in part presented at the Drug Delivery to the Lungs conference, Edinburgh, UK in 2018 and the Respiratory Drug Delivery conference virtually in 2020. The DPI formulation development, dissolution testing and PK study, as described elsewhere [10], were supported by FDA contracts HHSF223201110117A and HHSF223201610099C, and by grants 1U01FD004950 and 1U01FD005231 (to GH and JBB). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Food and Drug Administration (FDA).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors have no conflict on interest for the work presented in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Drescher, S.K., Jiao, Y., Chen, MJ. et al. Central and peripheral lung deposition of fluticasone propionate dry powder inhaler formulations in humans characterized by population pharmacokinetics. Pharm Res 40, 1177–1191 (2023). https://doi.org/10.1007/s11095-023-03472-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03472-6