Abstract
Objective
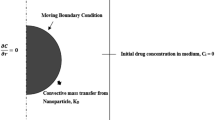
Customization of the rate of drug delivered based on individual patient requirements is of paramount importance in the design of drug delivery devices. Advances in manufacturing may enable multilayer drug delivery devices with different initial drug distributions in each layer. However, a robust mathematical understanding of how to optimize such capabilities is critically needed. The objective of this work is to determine the initial drug distribution needed in a spherical drug delivery device such as a capsule in order to obtain a desired drug release profile.
Methods
This optimization problem is posed as an inverse mass transfer problem, and optimization is carried out using the solution of the forward problem. Both non-erodible and erodible multilayer spheres are analyzed. Cases with polynomial forms of initial drug distribution are also analyzed. Optimization is also carried out for a case where an initial burst in drug release rate is desired, followed by a constant drug release rate.
Results
More than 60% reduction in root-mean-square deviation of the actual drug release rate from the ideal constant drug release rate is reported. Typically, the optimized initial drug distribution in these cases prevents or minimizes large drug release rate at early times, leading to a much more uniform drug release overall.
Conclusions
Results demonstrate potential for obtaining a desired drug delivery profile over time by carefully engineering the drug distribution in the drug delivery device. These results may help engineer devices that offer customized drug delivery by combining advanced manufacturing with mathematical optimization.











Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- A :
-
Initial (erodible case) or fixed (non-erodible case) radius (m).
- B :
-
Rate of erosion of the erodible sphere (m s−1).
- c :
-
Concentration (mol m−3).
- D :
-
Diffusion coefficient (m2 s−1).
- J :
-
Order of the polynomial function.
- M :
-
Number of layers.
- M total :
-
Total drug amount (mol).
- q a :
-
Actual drug release rate (mol s−1).
- q d :
-
Desired drug release rate (mol s−1).
- R :
-
Radius of erodible sphere as a function of time (m).
- r :
-
Radial coordinate (m).
- t :
-
Time (s).
- λ :
-
Non-dimensional eigenvalue.
- θ :
-
Non-dimensional concentration, θ = c/cref.
- τ :
-
Non-dimensional time, τ = Dt/A2
- ξ :
-
Non-dimensional radial coordinate, ξ = r/A
- in :
-
Initial
- ref :
-
Reference
- m :
-
Layer number
References
Siepmann J, Siepmann F. Mathematical modelling of drug delivery. Int J Pharm. 2008;364:328–43. https://doi.org/10.1016/j.ijpharm.2008.09.004.
Stefanini GG, Holmes DR. Drug-eluting coronary artery stents. N Engl J Med. 2013;368:254–65. https://doi.org/10.1056/NEJMra1210816.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8. https://doi.org/10.1038/nbt.1504.
Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for drug delivery systems. Annu Rev Chem Biomolec Eng. 2010;1:149–73. https://doi.org/10.1146/annurev-chembioeng-073009-100847.
Tarcha P. Polymers for controlled drug delivery. 1st ed: CRC Press; 1990.
Jain A, McGinty S, Pontrelli G, Zhou L. Theoretical model for diffusion-reaction based drug delivery from a multilayer spherical capsule. Int J Heat Mass Transf. 2022;183:122072:1-14. https://doi.org/10.1016/j.ijheatmasstransfer.2021.122072.
Laracuente M-L, Yu M, McHugh K. Zero-order drug delivery: state of the art and future prospects. J Control Release. 2020;327:834–56. https://doi.org/10.1016/j.jconrel.2020.09.020.
Bruschi ML. Mathematical models of drug release. Woodhead Publishing. 2015:63–86. https://doi.org/10.1016/B978-0-08-100092-2.00005-9.
Dekyndt B, Verin J, Neut C, Siepmann F, Siepmann J. How to easily provide zero order release of freely soluble drugs from coated pellets. Int J Pharm. 2015;478:31–8. https://doi.org/10.1016/j.ijpharm.2014.10.071.
Tzafriri AR, Groothuis A, Price GS, Edelman ER. Stent elution rate determines drug deposition and receptor-mediated effects. J Control Release. 2012;161:918–26. https://doi.org/10.1016/j.jconrel.2012.05.039.
Eyjolfsson, R., ‘Design and manufacture of pharmaceutical tablets,’ 1st Ed., Elsevier, 2014. ISBN: 9780128021828.
Vaithiyalingam SR, Sayeed VA. Critical factors in manufacturing multi-layer tablets—assessing material attributes, in-process controls, manufacturing process and product performance. Int J Pharm. 2010;398:9–13. https://doi.org/10.1016/j.ijpharm.2010.07.025.
Abebe A, Akseli I, Sprockel O, Kottala N, Cuitiño AM. Review of bilayer tablet technology. Int J Pharm. 2014;461:549–58. https://doi.org/10.1016/j.ijpharm.2013.12.028.
Goyanes A, Fina F, Martorana A, Sedough D, Gaisford S, Basit AW. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int J Pharm. 2014;527:21–30. https://doi.org/10.1016/j.ijpharm.2017.05.021.
Fina F, Madla CM, Goyanes A, Zhang J, Gaisford S, Basit AW. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int J Pharm. 2018;541:101–7. https://doi.org/10.1016/j.ijpharm.2018.02.015.
Karakurt I, Aydoğdu A, Çıkrıkcı S, Orozco J, Lin L. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: a controlled release study. Int J Pharm. 2020;584:119428. https://doi.org/10.1016/j.ijpharm.2020.119428.
Karalia D, Siamidi A, Karalis V, Vlachou M. 3D-printed Oral dosage forms: Mechanical properties, computational approaches and applications. Pharmaceutics. 2021;13:1401:1-37. https://doi.org/10.3390/pharmaceutics13091401.
Vergnaud J-M. Controlled drug release of oral dosage forms. Boca Raton, FL: CRC Press; 2009.
Arifin DY, Lee LY, Wang C-H. Mathematical modeling and simulation of drug release from microspheres: Implications to drug delivery systems. Adv Drug Deliv Rev. 2006;58:1274–325. https://doi.org/10.1016/j.addr.2006.09.007.
Crank J. The mathematics of diffusion. 2nd ed: Oxford Science Publications; 1980.
Jain A, McGinty S, Pontrelli G, Zhou L. Theoretical modeling of endovascular drug delivery into a multilayer Arterial Wall from a drug-coated balloon. Int J Heat Mass Transf. 2022;187:122572. https://doi.org/10.1016/j.ijheatmasstransfer.2022.122572.
d’Errico M, Sammarco P, Vairo G. Analytical modeling of drug dynamics induced by eluting stents in the coronary multi-layered curved domain. Math Biosci. 2015;267:79–96. https://doi.org/10.1016/j.mbs.2015.06.016.
Jain A, McGinty S, Pontrelli G. Drug diffusion and release from a bioerodible spherical capsule. Int J Pharm. 2022;616:121442. https://doi.org/10.1016/j.ijpharm.2021.121442.
Zhang M, Yang Z, Chow L-L, Wang C-H. Simulation of drug release from biodegradable polymeric microspheres with bulk and surface erosions. J Pharm Sci. 2003;92:2040–56. https://doi.org/10.1002/jps.10463.
Harland RS, Dubernet C, Benoit J-P, Peppas NA. A model of dissolution-controlled, diffusional drug release from non-swellable polymeric microspheres. J Control Release. 1988;7:207–15. https://doi.org/10.1016/0168-3659(88)90053-3.
Sundararaj SC, Thomas MV, Dziubla TD, Puleo DA. Bioerodible system for sequential release of multiple drugs. Acta Biomater. 2014;10:115–25. https://doi.org/10.1016/j.actbio.2013.09.031.
McGinty S, Pontrelli G. On the role of specific drug binding in modelling arterial eluting stents. J Math Chem. 2016;54:967–76. https://doi.org/10.1007/s10910-016-0618-7.
Özişik MN. Inverse heat transfer: fundamentals and applications. 1st ed. Routledge; 2000.
Lee PI. Initial concentration distribution as a mechanism for regulating drug release from diffusion controlled and surface erosion controlled matrix systems. J Control Release. 1986;4:1–7. https://doi.org/10.1016/0168-3659(86)90027-1.
Nauman EB, Patel K, Karande P. On the design and optimization of diffusion-controlled, planar delivery devices. Chem Eng Sci. 2010;65:923–30. https://doi.org/10.1016/j.ces.2009.09.043.
Georgiadis MC, Kostoglou M. On the optimization of drug release from multi-laminated polymer matrix devices. J Control Release. 2001;77:273–85. https://doi.org/10.1016/s0168-3659(01)00510-7.
Lu S, Ramirez F, Anseth KS. Modeling and optimization of drug release from laminated polymer matrix devices. AICHE J. 1998;44:1689–96. https://doi.org/10.1002/aic.690440720.
Larobina D, Mensitieri G, Kipper M, Narasimhan B. Mechanistic understanding of degradation in bioerodible polymers for drug delivery. AICHE J. 2002;48:2960–70. https://doi.org/10.1002/aic.690481221.
Wong HM, Wang JJ, Wang CH. In vitro release of human immunoglobulin G from biodegradable microspheres. Ind Eng Chem Res. 2001;40:933–48. https://doi.org/10.1021/ie0006256.
Nocedal J, Wright S Numerical optimization. Berlin/Heidelberg: Springer Science & Business Media; 2006.
Boyd S, Vandenberghe L. Convex optimization. Cambridge: Cambridge University Press; 2004.
Funding
Funding from the European Research Council under the European Unions Horizon 2020 Framework Programme (No. FP/2014 \ 0552020)/ ERC Grant Agreement No. 739964 (COPMAT) is acknowledged. This work is also partially supported by Italian MIUR (PRIN 2017 project: Mathematics of active materials: from mechanobiology to smart devices, # 2017KL4EF3).
Author information
Authors and Affiliations
Contributions
A. Jain – Conceptualization, Methodology, Formal Analysis, Validation, Investigation, Data Curation, Project Administration; K. Subbarao – Formal Analysis, Validation, Investigation, Data Curation; S. McGinty – Methodology, Formal Analysis, Validation; G. Pontrelli – Methodology, Formal Analysis, Validation. All authors contributed towards Writing Original Draft, Review and Editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have any conflicts of interest in connection with this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 258 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jain, A., Subbarao, K., McGinty, S. et al. Optimization of Initial Drug Distribution in Spherical Capsules for Personalized Release. Pharm Res 39, 2607–2620 (2022). https://doi.org/10.1007/s11095-022-03359-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03359-y