Abstract
Purpose
To evaluate the duration of effect of rHuPH20 on SC absorption of cetuximab and to develop a mechanistic pharmacokinetic model linking the kinetics of rHuPH20 action with hyaluronan (HA) homeostasis and absorption of cetuximab from the SC space.
Methods
Serum pharmacokinetics of cetuximab was evaluated after IV and SC dosing at 0.4 and 10 mg/kg (control groups). In test groups, SC cetuximab was administered simultaneously with rHuPH20 (Co-Injection) or 12 h after injection of rHuPH20 (Pre-Injection). Mechanistic pharmacokinetic model was developed to simultaneously capture cetuximab kinetics in all groups.
Results
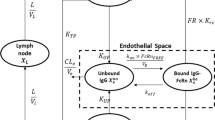
Administration of rHuPH20 resulted in a faster absorption of cetuximab; the difference between co-injection and pre-injection groups appeared to be dependent on the dose level. The model combined three major components: kinetics of rHuPH20 at SC site; HA homeostasis and its disruption by rHuPH20; and cetuximab systemic disposition and the effect of HA disruption on cetuximab SC absorption. The model provided good description of experimental data obtained in this study and collected previously.
Conclusions
Proposed model can serve as a potential translational framework for capturing the effect of rHuPH20 across multiple preclinical species and in human studies and can be used for optimization of SC delivery of biotherapeutics.









Similar content being viewed by others

References
Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507.
Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, Wu HC. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1.
Bittner B, Richter WF, Hourcade-Potelleret F, Herting F, Schmidt J. Non-clinical pharmacokinetic/pharmacodynamic and early clinical studies supporting development of a novel subcutaneous formulation for the monoclonal antibody rituximab. Drug Res (Stuttg). 2014;64(11):569–75.
Jackisch C, Muller V, Maintz C, Hell S, Ataseven B. Subcutaneous Administration of Monoclonal Antibodies in oncology. Geburtshilfe Frauenheilkd. 2014;74(4):343–9.
Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, Edgington TS, Shepard HM, Patton JS, Frost GI. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114(2):230–41.
Wasserman RL, Melamed I, Stein MR, Gupta S, Puck J, Engl W, Leibl H, McCoy B, Empson VG, Gelmont D, Schiff RI. Igsc wrSG. Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulins for primary immunodeficiency. J Allergy Clin Immunol. 2012;130(4):951–7 e911.
Bittner B, Richter W, Schmidt J. Subcutaneous Administration of Biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–40.
Alberts B. Essential cell biology : an introduction to the molecular biology of the cell. New York: Garland Pub; 1998.
Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92(3):1005–60.
Simpson MA, de la Motte C, Sherman LS, Weigel PH. Advances in Hyaluronan biology: signaling, regulation, and disease mechanisms. Int J Cell Biol. 2015;2015:690572.
Lee-Sayer SS, Dong Y, Arif AA, Olsson M, Brown KL, Johnson P. The where, when, how, and why of hyaluronan binding by immune cells. Front Immunol. 2015;6:150.
Henriksen JH. Degradation of bioactive substances : physiology and pathophysiology. Boca Raton, Fla: CRC Press; 1991.
Harris EN, Weigel JA, Weigel PH. Endocytic function, glycosaminoglycan specificity, and antibody sensitivity of the recombinant human 190-kDa hyaluronan receptor for endocytosis (HARE). J Biol Chem. 2004;279(35):36201–9.
Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4(4):427–40.
Hechter O. Reconstitution of the dermal barrier to fluid diffusion following administration of hyaluronidase. Proc Soc Exp Biol Med. 1948;67(3):343.
Wasserman RL. Recombinant human hyaluronidase-facilitated subcutaneous immunoglobulin infusion in primary immunodeficiency diseases. Immunotherapy. 2017;9(12):1035–50.
Carlson J, Cox K, Bedwell K, Ku M. Rituximab for subcutaneous delivery: clinical management principles from a nursing perspective. Int J Nurs Pract. 2015;21(Suppl 3):1–13.
Danieli MG, Pulvirenti F, Rocchi V, Morariu R, Quinti I. Self-administered hyaluronidase-facilitated subcutaneous immunoglobulin therapy in complicated primary antibody deficiencies. Immunotherapy. 2016;8(9):995–1002.
Wasserman RL, Melamed I, Kobrynski L, Puck J, Gupta S, Doralt J, Sharkhawy M, Engl W, Leibl H, Gelmont D, Yel L. Recombinant human hyaluronidase facilitated subcutaneous immunoglobulin treatment in pediatric patients with primary immunodeficiencies: long-term efficacy, safety and tolerability. Immunotherapy. 2016;8(10):1175–86.
Alberts BJA, Lewis J, et al. The Extracellular Matrix of Animals in Molecular Biology of the Cell. Garland Science. In: Molecular Biology of the Cell. New York: Garland Science; 2002.
Kang DW, Oh DA, Fu GY, Anderson JM, Zepeda ML. Porcine model to evaluate local tissue tolerability associated with subcutaneous delivery of protein. J Pharmacol Toxicol Methods. 2013;67(3):140–7.
Bittner B, Richter WF, Hourcade-Potelleret F, McIntyre C, Herting F, Zepeda ML, Schmidt J. Development of a subcutaneous formulation for trastuzumab - nonclinical and clinical bridging approach to the approved intravenous dosing regimen. Arzneimittelforschung. 2012;62(9):401–9.
Styles IK, Feeney OM, Nguyen TH, Brundel DHS, Kang DW, Clift R, McIntosh MP, Porter CJH. Removal of interstitial hyaluronan with recombinant human hyaluronidase improves the systemic and lymphatic uptake of cetuximab in rats. J Control Release. 2019;315:85–96.
Kagan L, Mager DE. Mechanisms of subcutaneous absorption of rituximab in rats. Drug Metab Dispos. 2013;41(1):248–55.
Galizia G, Lieto E, De Vita F, Orditura M, Castellano P, Troiani T, Imperatore V, Ciardiello F. Cetuximab, a chimeric human mouse anti-epidermal growth factor receptor monoclonal antibody, in the treatment of human colorectal cancer. Oncogene. 2007;26(25):3654–60.
Azzopardi N, Lecomte T, Ternant D, Boisdron-Celle M, Piller F, Morel A, Gouilleux-Gruart V, Vignault-Desvignes C, Watier H, Gamelin E, Paintaud G. Cetuximab pharmacokinetics influences progression-free survival of metastatic colorectal cancer patients. Clin Cancer Res. 2011;17(19):6329–37.
Tol J, Punt CJ. Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clin Ther. 2010;32(3):437–53.
Jadin L, Bookbinder LH, Frost GI. A comprehensive model of hyaluronan turnover in the mouse. Matrix Biol. 2012;31(2):81–9.
Connor RJ, Blouw B, Cowell J, Chen K, Zhao C, Kang DW. A preclinical investigation into the effects of aging on dermal Hyaluronan properties and reconstitution following recombinant human hyaluronidase PH20 administration. Dermatol Ther (Heidelb). 2020;10(3):503–13.
Armstrong SE, Bell DR. Relationship between lymph and tissue hyaluronan in skin and skeletal muscle. Am J Physiol Heart Circ Physiol. 2002;283(6):H2485–94.
Chapy H, Kagan L. Evaluation of the effects of animal growth and previous exposure on the pharmacokinetics of rituximab in rats. J Pharm Sci. 2018;107(7):1987–94.
Kagan L, Turner MR, Balu-Iyer SV, Mager DE. Subcutaneous absorption of monoclonal antibodies: role of dose, site of injection, and injection volume on rituximab pharmacokinetics in rats. Pharm Res. 2012;29(2):490–9.
Kagan L, Zhao J, Mager DE. Interspecies pharmacokinetic modeling of subcutaneous absorption of rituximab in mice and rats. Pharm Res. 2014;31(12):3265–73.
Necas J, Bartosikova L, Brauner P, Kolar J. Hyaluronic acid (hyaluronan): a review. Veterinarni Medicina. 2008;53:397–411.
Connor RJ, Taverna DM, Thrall K, LaBarre MJ, Kang DW. Use of computed tomography to assess subcutaneous drug dispersion with recombinant human hyaluronidase PH20 in a swine model. J Pharmacol Toxicol Methods. 2020;106:106936.
Almasa Bass AP, Mridha K, Sattler C, Kim AM, Plowchalk DR. Pharmacokinetics, pharmacodynamics, and safety of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, in healthy subjects when administered in co-mixture with recombinant human hyaluronidase: A phase 1 randomized trial. Health Sci Rep. 2018;1(9).
Luo FR, Yang Z, Dong H, Camuso A, McGlinchey K, Fager K, Flefleh C, Kan D, Inigo I, Castaneda S, Rose WC, Kramer RA, Wild R, Lee FY. Correlation of pharmacokinetics with the antitumor activity of Cetuximab in nude mice bearing the GEO human colon carcinoma xenograft. Cancer Chemother Pharmacol. 2005;56(5):455–64.
Cowman MK, Lee HG, Schwertfeger KL, McCarthy JB, Turley EA. The content and size of Hyaluronan in biological fluids and tissues. Front Immunol. 2015;6:261.
ACKNOWLEDGMENTS AND DISCLOSURES
The study was funded, in part, by a grant from Halozyme Therapeutics Inc. to Leonid Kagan. The authors would like to thank Dr. Charvi Nanavati for scientific discussions and support of the Rutgers-Halozyme collaboration. Xizhe Gao, JongBong Lee, Kiran Deshpande, Leonid Kagan – no conflict. David W Kang - employee of Halozyme Therapeutics Inc. Anas M Fathallah – former employee of Halozyme Therapeutics Inc.
Funding
The study was supported by a research grant from Halozyme Therapeutics Inc. to Leonid Kagan.
Author information
Authors and Affiliations
Contributions
Conception and design of the study – DWK, AMF, LK
Data acquisition – XG, JBL, KD
Data analysis and interpretation – XG, LK
Drafting the manuscript and critically revising – XG, LK, DWK, AMF
LK is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1.32 MB)
Rights and permissions
About this article
Cite this article
Gao, X., Lee, J., Deshpande, K. et al. Mechanistic Modeling of the Effect of Recombinant Human Hyaluronidase (rHuPH20) on Subcutaneous Delivery of Cetuximab in Rats. Pharm Res 39, 1867–1880 (2022). https://doi.org/10.1007/s11095-022-03294-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03294-y