Abstract
Purpose
Venetoclax (VEN), an anti-tumor drug that is a substrate of cytochrome P450 3A enzyme (CYP3A4), is used to treat leukemia. Voriconazole (VCZ) is an antifungal medication that inhibits CYP3A4. The goal of this study is to predict the effect of VCZ on VEN exposure.
Method
Two physiological based pharmacokinetics (PBPK) models were developed for VCZ and VEN using the bottom-up and top-down method. VCZ model was also developed to describe the effect of CYP2C19 polymorphism on its pharmacokinetics (PK). The reversible inhibition constant (Ki) of VCZ for CYP3A4 was calibrated using drug-drug interaction (DDI) data of midazolam and VCZ. The clinical verified VCZ and VEN model were used to predict the DDI of VCZ and VEN at clinical dosing scenario.
Result
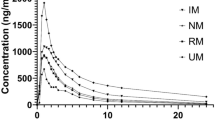
VCZ model predicted VCZ exposure in the subjects of different CYP2C19 genotype and DDI related fold changes of sensitive CYP3A substrate with acceptable prediction error. VEN model can capture PK of VEN with acceptable prediction error. The DDI PBPK model predicted that VCZ increased the exposure of VEN by 4.5–9.6 fold. The increase in VEN exposure by VCZ was influenced by subject’s CYP2C19 genotype. According to the therapeutic window, VEN dose should be reduced to 100 mg when co-administered with VCZ.
Conclusion
The PBPK model developed here could support individual dose adjustment of VEN and DDI risk assessment. Predictions using the robust PBPK model confirmed that the 100 mg dose adjustment is still applicable in the presence of VCZ with high inter-individual viability.





Similar content being viewed by others
Abbreviations
- AML:
-
Acute myeloid leukemia
- AUC:
-
The area under the concencention-time curve
- bid:
-
Twice daily
- CLL:
-
Chronic lymphocytic leukemia
- CYP:
-
Cytochrome P450
- CLint :
-
The enzymatic intrinsic clearance
- DDI:
-
Drug drug interaction
- EM:
-
Extensive metabolizer
- FMO:
-
Flavin-containing monooxygenase
- Fm :
-
Fraction of drugs metabolized
- IM:
-
Intermediate metabolizers
- Kcat :
-
Catalytic rate constant
- Ki :
-
Reversible inhibition constant
- Km :
-
Michaelis–Menten constant
- PBPK:
-
Physiologically based pharmacokinetics
- PM:
-
Poor metabolizer
- qd:
-
Once a day
- UM:
-
Ultrarapid metabolizer
- VCZ:
-
Voriconazole
- VEN:
-
Venetoclax
References
Venclexta (venetoclax) 2018 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208573s009lbl.pdf,[Accessed 11 Nov 2018];
Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006 Aug;91(8):1068–75.
Hahn-Ast C, Glasmacher A, Mückter S, Schmitz A, Kraemer A, Marklein G, et al. Overall survival and fungal infection-related mortality in patients with invasive fungal infection and neutropenia after myelosuppressive chemotherapy in a tertiary care centre from 1995 to 2006. J Antimicrob Chemother. 2010;65(4):761–8.
Pascual A, Csajka C, Buclin T, Bolay S, Bille J, Calandra T, et al. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin Infect Dis. 2012;55(3):381–90.
Agarwal SK, Hu B, Chien D, Wong SL, Salem AH. Evaluation of Rifampin’s transporter inhibitory and CYP3A inductive effects on the pharmacokinetics of venetoclax, a BCL-2 inhibitor: results of a single- and multiple-dose study. J Clin Pharmacol. 2016;56(11):1335–43.
Agarwal SK, Salem AH, Danilov AV, Hu B, Puvvada S, Gutierrez M, et al. Effect of ketoconazole, a strong CYP3A inhibitor, on the pharmacokinetics of venetoclax, a BCL-2 inhibitor, in patients with non-Hodgkin lymphoma. Br J Clin Pharmacol. 2017;83(4):846–54.
Freise KJ, Hu B, Salem AH. Impact of ritonavir dose and schedule on CYP3A inhibition and venetoclax clinical pharmacokinetics. Eur J Clin Pharmacol. 2018;74(4):413–21.
Jeong S, Nguyen PD, Desta Z. Comprehensive in vitro analysis of voriconazole inhibition of eight cytochrome P450 (CYP) enzymes: major effect on CYPs 2B6, 2C9, 2C19, and 3A. Antimicrob Agents Chemother. 2009;53(2):541–51.
Rausch CR, DiNardo CD, Maiti A, et al. Duration of cytopenias with concomitant venetoclax and azole antifungals in acute myeloid leukemia. Cancer. 2021;127:2489–99.
Yanni SB, Annaert PP, Augustijns P, Bridges A, Gao Y, Benjamin DK, et al. Role of flavin-containing monooxygenase in oxidative metabolism of voriconazole by human liver microsomes. Drug Metab Dispos. 2008;36(6):1119–25.
Scholz I, Oberwittler H, Riedel K-D, Burhenne J, Weiss J, Haefeli WE, et al. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br J Clin Pharmacol. 2009;68(6):906–15.
Saari TI, Laine K, Leino K, Valtonen M, Neuvonen PJ, Olkkola KT. Effect of voriconazole on the pharmacokinetics and pharmacodynamics of intravenous and oral midazolam. Clin Pharmacol Ther. 2006;79(4):362–70.
Saari TI, Laine K, Leino K, Valtonen M, Neuvonen PJ, Olkkola KT. Voriconazole, but not terbinafine, markedly reduces alfentanil clearance and prolongs its half-life. Clin Pharmacol Ther. 2006;80(5):502–8.
Hanke N, Frechen S, Moj D, Britz H, Eissing T, Wendl T, et al. PBPK models for CYP3A4 and P-gp DDI prediction: a modeling network of rifampicin, itraconazole, clarithromycin, midazolam, alfentanil, and digoxin. CPT Pharmacometrics Syst Pharmacol. 2018;7(10):647–59.
Cheung TT, Salem AH, Menon RM, Munasinghe WP, Bueno OF, Agarwal SK. Pharmacokinetics of the BCL-2 inhibitor venetoclax in healthy chinese subjects. Clin Pharmacol Drug Dev. 2018;7(4):435–40.
Agarwal SK, DiNardo CD, Potluri J, Dunbar M, Kantarjian HM, Humerickhouse RA, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther. 2017;39(2):359–67.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45(10):1013–34.
Mordenti J. Man versus beast: pharmacokinetic scaling in mammals. J Pharm Sci. 1986;75(11):1028–40.
Li X, Frechen S, Moj D, Lehr T, Taubert M, Hsin C-H, et al. A physiologically based pharmacokinetic model of voriconazole integrating time-dependent inhibition of CYP3A4, genetic polymorphisms of CYP2C19 and predictions of drug-drug interactions. Clin Pharmacokinet. 2020;59(6):781–808.
Emami Riedmaier A, Lindley DJ, Hall JA, Castleberry S, Slade RT, Stuart P, et al. Mechanistic physiologically based pharmacokinetic modeling of the dissolution and food effect of a biopharmaceutics classification system iv compound-the venetoclax story. J Pharm Sci. 2018;107(1):495–502.
Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob Agents Chemother. 2002;46(8):2546–53.
Damle B, Varma MV, Wood N. Pharmacokinetics of voriconazole administered concomitantly with fluconazole and population-based simulation for sequential use. Antimicrob Agents Chemother. 2011;55(11):5172–7.
Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31(5):540–7.
Michaelis L, Menten ML, Johnson KA, Goody RS. The original Michaelis constant: translation of the 1913 Michaelis-Menten paper. Biochemistry. 2011;50(39):8264–9.
Mikus G, Schöwel V, Drzewinska M, Rengelshausen J, Ding R, Riedel K-D, et al. Potent cytochrome P450 2C19 genotype-related interaction between voriconazole and the cytochrome P450 3A4 inhibitor ritonavir. Clin Pharmacol Ther. 2006;80(2):126–35.
Lee S, Kim B-H, Nam W-S, Yoon SH, Cho J-Y, Shin S-G, et al. Effect of CYP2C19 polymorphism on the pharmacokinetics of voriconazole after single and multiple doses in healthy volunteers. J Clin Pharmacol. 2012;52(2):195–203.
Meyer M, Schneckener S, Ludewig B, Kuepfer L, Lippert J. Using expression data for quantification of active processes in physiologically based pharmacokinetic modeling. Drug Metab Dispos. 2012;40(5):892–901.
Rodrigues AD. Integrated cytochrome P450 reaction phenotyping: attempting to bridge the gap between cDNA-expressed cytochromes P450 and native human liver microsomes. Biochem Pharmacol. 1999;57(5):465–80.
Steere B, Baker JAR, Hall SD, Guo Y. Prediction of in vivo clearance and associated variability of CYP2C19 substrates by genotypes in populations utilizing a pharmacogenetics-based mechanistic model. Drug Metab Dispos. 2015;43(6):870–83.
FDA. Vfend (voriconazole) 2010 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021266s032lbl.pdf,[Accessed 6 Dec 2010];
Freise KJ, Shebley M, Salem AH. Quantitative prediction of the effect of CYP3A inhibitors and inducers on venetoclax pharmacokinetics using a physiologically based pharmacokinetic model. J Clin Pharmacol. 2017;57(6):796–804.
Venetoclax “Clinical pharmacology & Biopharmaceutics review. 2016 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208573Orig1s000 ClinPharmR.pdf,[Accessed;
Krishna G, Ma L, Martinho M, Preston RA, O’Mara E. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J Antimicrob Chemother. 2012;67(11):2725–30.
FDA. Noxafil 2016 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022003s018s020,0205053s002s004,0205596s001s003lbl.pdf 22 Jan 2016];
Krishna G, Ma L, Prasad P, Moton A, Martinho M, O’Mara E. Effect of posaconazole on the pharmacokinetics of simvastatin and midazolam in healthy volunteers. Expert Opin Drug Metab Toxicol. 2012;8(1):1–10.
Salem AH, Agarwal SK, Dunbar M, Enschede SLH, Humerickhouse RA, Wong SL. Pharmacokinetics of venetoclax, a novel BCL-2 Inhibitor, in patients with relapsed or refractory chronic lymphocytic leukemia or non-hodgkin lymphoma. J Clin Pharmacol. 2017;57(4):484–92.
Lam YWF, Alfaro CL, Ereshefsky L, Miller M. Pharmacokinetic and pharmacodynamic interactions of oral midazolam with ketoconazole, fluoxetine, fluvoxamine, and nefazodone. J Clin Pharmacol. 2003;43(11):1274–82.
Boulenc X, Nicolas O, Hermabessière S, Zobouyan I, Martin V, Donazzolo Y, et al. CYP3A4-based drug-drug interaction: CYP3A4 substrates’ pharmacokinetic properties and ketoconazole dose regimen effect. Eur J Drug Metab Pharmacokinet. 2016;41(1):45–54.
Bhatnagar S, Mukherjee D, Salem AH, Miles D, Menon RM, Gibbs JP. Dose adjustment of venetoclax when co-administered with posaconazole: clinical drug-drug interaction predictions using a PBPK approach. Cancer Chemother Pharmacol. 2021;87(4):465–74.
Shirasaka Y, Chaudhry AS, McDonald M, Prasad B, Wong T, Calamia JC, et al. Interindividual variability of CYP2C19-catalyzed drug metabolism due to differences in gene diplotypes and cytochrome P450 oxidoreductase content. Pharmacogenomics J. 2016;16(4):375–87.
Yamazaki H, Nakamoto M, Shimizu M, Murayama N, Niwa T. Potential impact of cytochrome P450 3A5 in human liver on drug interactions with triazoles. Br J Clin Pharmacol. 2010;69(6):593–7.
Weiss J, Ten Hoevel MM, Burhenne J, Walter-Sack I, Hoffmann MM, Rengelshausen J, et al. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49(2):196–204.
Moriyama B, Obeng AO, Barbarino J, Penzak SR, Henning SA, Scott S, Agundez J, Wingard JR, McLeod HL, Klein TE, et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin Pharmacol Ther. 2016;102:45–51.
Jin H, Wang T, Falcione BA, Olsen KM, Chen K, Tang H, et al. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother. 2016;71(7):1772–85.
Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46(2):201–11.
Hamada Y, Tokimatsu I, Mikamo H, Kimura M, Seki M, Takakura S, et al. Practice guidelines for therapeutic drug monitoring of voriconazole: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother. 2013;19(3):381–92.
Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull. 2005;28:1805–8.
Acknowledgments
The authors would like to thank Jae-Gook Shin, Zi-teng Wang for helpful discussion on topics related to the work.
Funding
This work was supported by National Clinical Research Center for Hemotologic Diseases, the First Affiliated Hospital of Soochow Univerisity(2020WSC07).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
We confirm that the manuscript has been read and approved by all named authors and there are no other persons who satisfied the criteria for authorship but are not listed.
Conflict of Interest
We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that there are no known conflicts of interest associated with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, J., Liu, Sb., Rasheduzzaman, J.M. et al. Development of Physiology Based Pharmacokinetic Model to Predict the Drug Interactions of Voriconazole and Venetoclax. Pharm Res 39, 1921–1933 (2022). https://doi.org/10.1007/s11095-022-03289-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03289-9