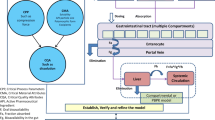
Abstract
The use of physiologically based pharmacokinetic (PBPK) modeling to support the drug product quality attributes, also known as physiologically based biopharmaceutics modeling (PBBM) is an evolving field and the interest in using PBBM is increasing. The US-FDA has emphasized on the use of patient centric quality standards and clinically relevant drug product specifications over the years. Establishing an in vitro in vivo link is an important step towards achieving the goal of patient centric quality standard. Such a link can aid in constructing a bioequivalence safe space and establishing clinically relevant drug product specifications. PBBM is an important tool to construct a safe space which can be used during the drug product development and lifecycle management. There are several advantages of using the PBBM approach, though there are also a few challenges, both with in vitro methods and in vivo understanding of drug absorption and disposition, that preclude using this approach and therefore further improvements are needed. In this review we have provided an overview of experience gained so far and the current perspective from regulatory and industry point of view. Collaboration between scientists from regulatory, industry and academic fields can further help to advance this field and deliver on promises that PBBM can offer towards establishing patient centric quality standards.






Similar content being viewed by others
References
Wagner JG. Biopharmaceutics: Gastrointestinal Absorption Aspects. Antibiot Chemother. 1964;12:53–84.
Wagner JG. Biopharmaceutics: absorption aspects. J Pharm Sci. 1961;50:359–87.
Ho NFH, Merkle HP, Higuchi WI. Quantitative, mechanistic and physiologically realistic approach to the biopharmaceutical design of oral drug delivery systems. Drug Dev Ind Pharm. 1983;9(7):1111–84.
Dressman JB, Fleisher D, Amidon GL. Physicochemical model for dose-dependent drug absorption. J Pharm Sci. 1984;73(9):1274–9.
Dressman JB, Fleisher D. Mixing-tank model for predicting dissolution rate control or oral absorption. J Pharm Sci. 1986;75(2):109–16.
Sinko PJ, Leesman GD, Amidon GL. Predicting fraction dose absorbed in humans using a macroscopic mass balance approach. Pharm Res. 1991;8(8):979–88.
Yu LX, Crison JR, Amidon GL. Compartmental transit and dispersion model analysis of small intestinal transit flow in humans. Int J Pharm. 1996;140(1):111–8.
Yu LX, Lipka E, Crison JR, Amidon GL. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv Drug Deliv Rev. 1996;19(3):359–76.
Yu LX, Amidon GL. Characterization of small intestinal transit time distribution in humans. Int J Pharm. 1998;171(2):157–63.
Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm. 1999;186(2):119–25.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50(Suppl 1):S41-67.
Jamei M. Recent Advances in Development and Application of Physiologically-Based Pharmacokinetic (PBPK) Models: a Transition from Academic Curiosity to Regulatory Acceptance. Curr Pharmacol Rep. 2016;2:161–9.
Stamatis SD, Rose JP. Lilly Absorption Modeling Platform: A Tool for Early Absorption Assessment. Mol Pharm. 2022;19(1):213–26.
Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:300–21.
Mudie DM, Murray K, Hoad CL, Pritchard SE, Garnett MC, Amidon GL, et al. Quantification of gastrointestinal liquid volumes and distribution following a 240 mL dose of water in the fasted state. Mol Pharm. 2014;11(9):3039–47.
Hens B, Bolger MB. Application of a Dynamic Fluid and pH Model to Simulate Intraluminal and Systemic Concentrations of a Weak Base in GastroPlus(). J Pharm Sci. 2019;108(1):305–15.
Zhang X, Lionberger RA, Davit BM, Yu LX. Utility of physiologically based absorption modeling in implementing Quality by Design in drug development. AAPS J. 2011;13(1):59–71.
Willmann S, Schmitt W, Keldenich J, Lippert J, Dressman JB. A physiological model for the estimation of the fraction dose absorbed in humans. J Med Chem. 2004;47(16):4022–31.
Abend A, Heimbach T, Cohen M, Kesisoglou F, Pepin X, Suarez-Sharp S. Dissolution and Translational Modeling Strategies Enabling Patient-Centric Drug Product Development: the M-CERSI Workshop Summary Report. The AAPS journal. 2018;20(3).
Pepin XJH, Parrott N, Dressman J, Delvadia P, Mitra A, Zhang X, et al. Current State and Future Expectations of Translational Modeling Strategies to Support Drug Product Development, Manufacturing Changes and Controls: A Workshop Summary Report. J Pharm Sci. 2021;110:555–66.
Mitra A, Suarez-Sharp S, Pepin XJH, Flanagan T, Zhao Y, Kotzagiorgis E, et al. Applications of Physiologically Based Biopharmaceutics Modeling (PBBM) to Support Drug Product Quality: A Workshop Summary Report. J Pharm Sci. 2021;110(2):594–609.
Hens B, Sinko PD, Job N, Dean M, Al-Gousous J, Salehi N, et al. Formulation predictive dissolution (fPD) testing to advance oral drug product development: An introduction to the US FDA funded '21st Century BA/BE' project. Int J Pharm. 2018;548(1):120–7.
Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011;89(2):259–67.
U. S. Food and Drug Administration. Draft Guidance for Industry: The Use of Physiologically Based Pharmacokinetic Analyses — Biopharmaceutics Applications for Oral Drug Product Development, Manufacturing Changes, and Controls. 2020. Available from: https://www.fda.gov/media/142500/download. Accessed 15 Dec 2021.
Tsakalozou E, Alam K, Babiskin A, Zhao L. Physiologically-Based Pharmacokinetic Modeling to Support Determination of Bioequivalence for Dermatological Drug Products: Scientific and Regulatory Considerations. Clin Pharmacol Ther. 2021.
The Center for Research on Complex Generics (CRCG): The University of Maryland, Baltimore and University of Michigan. 2021. Available from: http://www.complexgenerics.org/PBPK2021/.
Suarez-Sharp S, Cohen M, Kesisoglou F, Abend A, Marroum P, Delvadia P, et al. Applications of Clinically Relevant Dissolution Testing: Workshop Summary Report. AAPS J. 2018;20(6):93.
Heimbach T, Suarez-Sharp S, Kakhi M, Holmstock N, Olivares-Morales A, Pepin X, et al. Dissolution and Translational Modeling Strategies Toward Establishing an In Vitro-In Vivo Link—a Workshop Summary Report. The AAPS Journal. 2019;21(2).
U.S FDA CDER. CDER patient-focused drug development. [Available from: https://www.fda.gov/drugs/development-approval-process-drugs/cder-patient-focused-drug-development].
J W. The concept of pharmaceutical quality. American Pharmaceutical Review. 2004;7(6):10–5.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16(4):771–83.
Anand O. Clinically Relevant Dissolution Specifications a Biopharmaceutics’ Risk Based Approach: an FDA perspective: The Academy of Pharmaceutical Sciences; 2021 [Available from: https://www.apsgb.co.uk/wp-content/uploads/2021/05/Clinically-Relevant-Dissolution-Specifications-an-FDA-Perspective-__Om-Anand.pdf.
Raines K. PBPK Biopharmaceutics Guidance and progress on Risk Assessment 2021 [Available from: http://www.complexgenerics.org/media/SOP/complexgenerics/pdf/Conference-Slides/D2-04%20Kimberly%20Raines_PBPKGuidanceRiskAssessment.pdf
Parrott N, Hainzl D, Scheubel E, Krimmer S, Boetsch C, Guerini E, et al. Physiologically Based Absorption Modelling to Predict the Impact of Drug Properties on Pharmacokinetics of Bitopertin. AAPS J. 2014;16(5):1077–84.
Paul S. Challenges and strategies in establishing an in-vitro invivo link, in Dissolution and translational modeling strategies enabling patient centric product development. 2017.
U. S. Food and Drug Administration. Guidance for Industry: Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations. 1997. Available at: https://www.fda.gov/media/70939/download. Accessed 15 Dec 2021.
Wu F, Shah H, Li M, Duan P, Zhao P, Suarez S, et al. Biopharmaceutics Applications of Physiologically Based Pharmacokinetic Absorption Modeling and Simulation in Regulatory Submissions to the US Food and Drug Administration for New Drugs. AAPS J. 2021;23(2):1–14.
Heimbach T, Kesisoglou F, Novakovic J, Tistaert C, Mueller-Zsigmondy M, Kollipara S, et al. Establishing the Bioequivalence Safe Space for Immediate-Release Oral Dosage Forms using Physiologically Based Biopharmaceutics Modeling (PBBM): Case Studies. J Pharm Sci. 2021;110(12):3896–906.
Kato T, Nakagawa H, Mikkaichi T, Miyano T, Matsumoto Y, Ando S. Establishment of a clinically relevant specification for dissolution testing using physiologically based pharmacokinetic (PBPK) modeling approaches. Eur J Pharm Biopharm. 2020;151:45–52.
Zhao Y SS. FDA expectations in building a safe space to gain regulatory flexibility based on PBBM. 2019. Available from: https://cersi.umd.edu/sites/cersi.umd.edu/files/Day%203-1%20Zhao%20Suarez%20LM.pdf.
Tistaert C. Case Study: Bridging physiology-based dissolution testing to quality control testing using PBBM University of Maryland, College Park, MD2019 [Available from: https://cersi.umd.edu/file/day-3-5-christophe-tistaert-finalpdf.
Fan J, Zhang X, Zhao L. Utility of Physiologically Based Pharmacokinetic Absorption Modeling to Predict the Impact of Salt-to-Base Conversion on Prasugrel HCl Product Bioequivalence in the Presence of Proton Pump Inhibitors. AAPS J. 2017;19(5):1479–86.
Butler J, Hens B, Vertzoni M, Brouwers J, Berben P, Dressman J, et al. In vitro models for the prediction of in vivo performance of oral dosage forms: Recent progress from partnership through the IMI OrBiTo collaboration. Eur J Pharm Biopharm. 2019;136:70–83.
Taylor LS, Zhang GGZ. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv Drug Deliv Rev. 2016;101:122–42.
Heimbach T, Kesisoglou F, Novakovic J, Tistaert C, Mueller-Zsigmondy M, Kollipara S, et al. Establishing the Bioequivalence Safe Space for Immediate-Release Oral Dosage Forms using Physiologically Based Biopharmaceutics Modeling (PBBM): Case Studies. Journal of Pharmaceutical Sciences. 2021.
Parrott N, Suarez-Sharp S, Kesisoglou F, Pathak SM, Good D, Wagner C, et al. Best Practices in the Development and Validation of Physiologically Based Biopharmaceutics Modeling. A Workshop Summary Report. J Pharm Sci. 2021;110(2):584–93.
Delvadia PR, Barr WH, Karnes HT. A biorelevant in vitro release/permeation system for oral transmucosal dosage forms. Int J Pharm. 2012;430(1–2):104–13.
Willmann S, Thelen K, Lippert J. Integration of dissolution into physiologically-based pharmacokinetic models III: PK-Sim(R). J Pharm Pharmacol. 2012;64(7):997–1007.
Elagolix Tablets, NDA 210450 Food and Drug Administration. 2018. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210450Orig1s000ChemR.pdf].
Miao L, Mousa YM, Zhao L, Raines K, Seo P, Wu F. Using a Physiologically Based Pharmacokinetic Absorption Model to Establish Dissolution Bioequivalence Safe Space for Oseltamivir in Adult and Pediatric Populations. AAPS J. 2020;22(5):107.
Suarez-Sharp S, Li M, Duan J, Shah H, Seo P. Regulatory Experience with In Vivo In Vitro Correlations (IVIVC) in New Drug Applications. AAPS J. 2016;18(6):1379–90.
Triclabendazole Tablets, NDA 208711, Food and Drug Administration. 2018. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/208711Orig1s000ChemR.pdf].
Pepin XJ, Flanagan TR, Holt DJ, Eidelman A, Treacy D, Rowlings CE. Justification of Drug Product Dissolution Rate and Drug Substance Particle Size Specifications Based on Absorption PBPK Modeling for Lesinurad Immediate Release Tablets. Mol Pharm. 2016;13(9):3256–69.
Duvelisib Capsules, NDA211155, Food and Drug Administration. 2018. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211155Orig1Orig2s000ChemR.pdf].
Kesisoglou F, Wang M, Galipeau K, Harmon P, Okoh G, Xu W. Effect of Amorphous Nanoparticle Size on Bioavailability of Anacetrapib in Dogs. J Pharm Sci. 2019;108(9):2917–25.
Đuranović M, Madžarević M, Ivković B, Ibrić S, Cvijić S. The evaluation of the effect of different superdisintegrants on the drug release from FDM 3D printed tablets through different applied strategies: In vitro-in silico assessment. Int J Pharm. 2021;610.
Cámara-Martinez I, Blechar JA, Ruiz-Picazo A, Garcia-Arieta A, Calandria C, Merino-Sanjuan V, et al. Level A IVIVC for immediate release tablets confirms in vivo predictive dissolution testing for ibuprofen. Int J Pharm. 2022;614.
Kesisoglou F, Mitra A. Application of Absorption Modeling in Rational Design of Drug Product Under Quality-by-Design Paradigm. AAPS J. 2015;17(5):1224–36.
Pepin XJH, Moir AJ, Mann JC, Sanderson NJ, Barker R, Meehan E, et al. Bridging in vitro dissolution and in vivo exposure for acalabrutinib. Part II. A mechanistic PBPK model for IR formulation comparison, proton pump inhibitor drug interactions, and administration with acidic juices. European journal of pharmaceutics and biopharmaceutics. 2019;142:435–48.
Dickinson PA, Lee WW, Stott PW, Townsend AI, Smart JP, Ghahramani P, et al. Clinical Relevance of Dissolution Testing in Quality by Design. AAPS J. 2008;10(2):380–90.
Jereb R, Kristl A, Mitra A. Prediction of fasted and fed bioequivalence for immediate release drug products using physiologically based biopharmaceutics modeling (PBBM). Eur J Pharm Sci. 2020;155:105554.
Stillhart C, Pepin X, Tistaert C, Good D, Van Den Bergh A, Parrott N, et al. PBPK Absorption Modeling: Establishing the In Vitro–In Vivo Link—Industry Perspective. The AAPS Journal. 2019;21(2).
Loisios-Konstantinidis I, Cristofoletti R, Fotaki N, Turner DB, Dressman J. Establishing virtual bioequivalence and clinically relevant specifications using in vitro biorelevant dissolution testing and physiologically-based population pharmacokinetic modeling. case example: Naproxen. European Journal of Pharmaceutical Sciences. 2020;143:105170.
Macwan JS, Fraczkiewicz G, Bertolino M, Krüger P, Peters S-A. Application of physiologically based biopharmaceutics modeling to understand the impact of dissolution differences on in vivo performance of immediate release products: The case of bisoprolol. 2021;10(6):622-32.
Farhan N, Cristofoletti R, Basu S, Kim S, Lingineni K, Jiang S, et al. Physiologically-based pharmacokinetics modeling to investigate formulation factors influencing the generic substitution of dabigatran etexilate. 2021;10(3):199-210
García MA, Bolger MB, Suarez-Sharp S, Langguth P. Predicting pharmacokinetics of multisource acyclovir oral products through physiologically based biopharmaceutics modeling. Journal of Pharmaceutical Sciences. 2021.
Jaiswal S, Ahmed T, Kollipara S, Bhargava M, Chachad S. Development, validation and application of Physiologically Based Biopharmaceutics Model to justify the change in dissolution specifications for DRL ABC Extended release tablets. Drug Development and Industrial Pharmacy. 2021(just-accepted):1–31.
Wu X, Zhang X, Xu R, Shaik IH, Venkataramanan R. Physiologically based pharmacokinetic modelling of treprostinil after intravenous injection and extended‐release oral tablet administration in healthy volunteers: An extrapolation to other patient populations including patients with hepatic impairment. British Journal of Clinical Pharmacology. 2021.
Matsui K, Takeuchi S, Haruna Y, Yamane M, Shimizu T, Hatsuma Y, et al. Transverse comparison of mannitol content in marketed drug products: Implication for no-effect dose of sugar alcohols on oral drug absorption. Journal of Drug Delivery Science and Technology. 2020;57:101728.
Yamane M, Matsui K, Sugihara M, Tokunaga Y. The Provisional No-Effect Threshold of Sugar Alcohols on Oral Drug Absorption Estimated by Physiologically Based Biopharmaceutics Model. J Pharm Sci. 2021;110(1):467–77.
Jamei M, Abrahamsson B, Brown J, Bevernage J, Bolger MB, Heimbach T, et al. Current status and future opportunities for incorporation of dissolution data in PBPK modeling for pharmaceutical development and regulatory applications: OrBiTo consortium commentary. Eur J Pharm Biopharm. 2020;155:55–68.
Klumpp L, Dressman J. Physiologically based pharmacokinetic model outputs depend on dissolution data and their input: Case examples glibenclamide and dipyridamole. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2020;151:105380.
Takano R, Sugano K, Higashida A, Hayashi Y, Machida M, Aso Y, et al. Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharm Res. 2006;23(6):1144–56.
Pohl P, Saparov SM, Antonenko YN. The Size of the Unstirred Layer as a Function of the Solute Diffusion Coefficient. Biophys J. 1998;75(3):1403–9.
Hofsäss MA, Dressman J. Suitability of the z-Factor for Dissolution Simulation of Solid Oral Dosage Forms: Potential Pitfalls and Refinements. J Pharm Sci. 2020;109(9):2735–45.
Pepin X, Blanchon S, Couarraze G. Powder dynamic contact angle data in the pharmaceutical industry. Pharm Sci Technolo Today. 1999;2(3):111–8.
Pathak SM, Ruff A, Kostewicz ES, Patel N, Turner DB, Jamei M. Model-Based Analysis of Biopharmaceutic Experiments To Improve Mechanistic Oral Absorption Modeling: An Integrated in Vitro in Vivo Extrapolation Perspective Using Ketoconazole as a Model Drug. Mol Pharm. 2017;14(12):4305–20.
Pepin XJH, Sanderson NJ, Blanazs A, Grover S, Ingallinera TG, Mann JC. Bridging in vitro dissolution and in vivo exposure for acalabrutinib. Part I Mechanistic modelling of drug product dissolution to derive a P-PSD for PBPK model input. European Journal of Pharmaceutics and Biopharmaceutics. 2019;142:421–34.
Pepin X, Goetschy M, Abrahmsén-Alami S. Mechanistic models for USP2 dissolution apparatus, including fluid hydrodynamics and sedimentation. Journal of Pharmaceutical Sciences. 2021.
Laisney M, Heimbach T, Mueller-Zsigmondy M, Blumenstein L, Costa R, Ji Y. Physiologically Based Biopharmaceutics Modeling to Demonstrate Virtual Bioequivalence and Bioequivalence Safe-space for Ribociclib which has Permeation Rate-controlled Absorption. Journal of Pharmaceutical Sciences. 2021.
Kesisoglou F, Chung J, van Asperen J, Heimbach T. Physiologically Based Absorption Modeling to Impact Biopharmaceutics and Formulation Strategies in Drug Development-Industry Case Studies. J Pharm Sci. 2016;105(9):2723–34.
Duan JZ. A Biopharmetrics Approach for Drug Product Quality Control with Clinical Relevance. J Pharm Sci. 2021;110(1):478–88.
Pepin X, editor Use of IVIVc and IVIVe to support formulation development - Industrial case studies. AAPS meeting - 4th November 2014; 2014; San Diego, USA.
Stillhart C, Parrott NJ, Lindenberg M, Chalus P, Bentley D, Szepes A. Characterising Drug Release from Immediate-Release Formulations of a Poorly Soluble Compound, Basmisanil, Through Absorption Modelling and Dissolution Testing. AAPS J. 2017;19(3):827–36.
Watson KJ, Davis J, Jones HM. Application of Physiologically Based Pharmacokinetic Modeling to Understanding the Clinical Pharmacokinetics of UK-369,003. Drug Metab Dispos. 2011;39(7):1203–13.
Ni Z, Talattof A, Fan J, Tsakalozou E, Sharan S, Sun D, et al. Physiologically Based Pharmacokinetic and Absorption Modeling for Osmotic Pump Products. AAPS J. 2017;19(4):1045–53.
Schiller C, Frohlich CP, Giessmann T, Siegmund W, Monnikes H, Hosten N, et al. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2005;22(10):971–9.
Sutton SC. Role of physiological intestinal water in oral absorption. AAPS J. 2009;11(2):277–85.
Valentin J. Basic anatomical and physiological data for use in radiological protection: reference values. Ann ICRP. 2002;32(3–4):1–277.
Kesisoglou F, Xia B, Agrawal NGB. Comparison of Deconvolution-Based and Absorption Modeling IVIVC for Extended Release Formulations of a BCS III Drug Development Candidate. AAPS J. 2015;17(6):1492–500.
Eckernäs E, Tannergren C. Physiologically Based Biopharmaceutics Modeling of Regional and Colon Absorption in Dogs. Mol Pharm. 2021;18(4):1699–710.
Paraiso RL, Rose RH, Fotaki N, McAllister M, Dressman JB. The use of PBPK/PD to establish clinically relevant dissolution specifications for zolpidem immediate release tablets. Eur J Pharm Sci. 2020;155:105534.
Brown J, Chien C, Timmins P, Dennis A, Doll W, Sandefer E, et al. Compartmental absorption modeling and site of absorption studies to determine feasibility of an extended-release formulation of an HIV-1 attachment inhibitor phosphate ester prodrug. J Pharm Sci. 2013;102(6):1742–51.
Salehi N, Al-Gousous J, Mudie DM, Amidon GL, Ziff RM, Amidon GE. Hierarchical Mass Transfer Analysis of Drug Particle Dissolution, Highlighting the Hydrodynamics, pH, Particle Size, and Buffer Effects for the Dissolution of Ionizable and Nonionizable Drugs in a Compendial Dissolution Vessel. Mol Pharm. 2020;17(10):3870–84.
Worsoe J, Fynne L, Gregersen T, Schlageter V, Christensen LA, Dahlerup JF, et al. Gastric transit and small intestinal transit time and motility assessed by a magnet tracking system. BMC Gastroenterol. 2011;11:145.
Koziolek M, Grimm M, Schneider F, Jedamzik P, Sager M, Kühn JP, et al. Navigating the human gastrointestinal tract for oral drug delivery: Uncharted waters and new frontiers. Adv Drug Deliv Rev. 2016;101:75–88.
Rao SSC, Sadeghi P, Beaty J, Kavlock R. Ambulatory 24-Hour Colonic Manometry in Slow-Transit Constipation. Am J Gastroenterol. 2004;99(12):2405–16.
Mendes TC, Simon A, Menezes JCV, Pinto EC, Cabral LM, de Sousa VP. Development of USP Apparatus 3 Dissolution Method with IVIVC for Extended Release Tablets of Metformin Hydrochloride and Development of a Generic Formulation. Chem Pharm Bull (Tokyo). 2019;67(1):23–31.
Schick P, Sager M, Wegner F, Wiedmann M, Schapperer E, Weitschies W, et al. Application of the GastroDuo as an in Vitro Dissolution Tool To Simulate the Gastric Emptying of the Postprandial Stomach. Mol Pharm. 2019;16(11):4651–60.
Hopgood M, Reynolds G, Barker R. Using Computational Fluid Dynamics to Compare Shear Rate and Turbulence in the TIM-Automated Gastric Compartment With USP Apparatus II. J Pharm Sci. 2018;107(7):1911–9.
Barker R, Abrahamsson B, Kruusmägi M. Application and Validation of an Advanced Gastrointestinal In Vitro Model for the Evaluation of Drug Product Performance in Pharmaceutical Development. J Pharm Sci. 2014;103(11):3704–12.
Bermejo M, Hens B, Dickens J, Mudie D, Paixão P, Tsume Y, et al. A Mechanistic Physiologically-Based Biopharmaceutics Modeling (PBBM) Approach to Assess the In Vivo Performance of an Orally Administered Drug Product: From IVIVC to IVIVP. Pharmaceutics. 2020;12(1).
Li M, Zhang X, Wu D, Anand O, Chen H, Raines K, et al. Understanding In Vivo Dissolution of Immediate Release (IR) Solid Oral Drug Products Containing Weak Acid BCS Class 2 (BCS Class 2a) Drugs. AAPS J. 2021;23(6):113.
Komasaka T, Dressman J. Simulation of oral absorption from non-bioequivalent dosage forms of the salt of raltegravir, a poorly soluble acidic drug, using a physiologically based biopharmaceutical modeling (PBBM) approach. Eur J Pharm Sci. 2021;157:105630.
Langguth P, Lee KM, Spahn-Langguth H, Amidon GL. Variable gastric emptying and discontinuities in drug absorption profiles: Dependence of rates and extent of cimetidine absorption on motility phase and pH. Biopharm Drug Dispos. 1994;15(9):719–46.
Andreas CJ, Pepin X, Markopoulos C, Vertzoni M, Reppas C, Dressman J. Mechanistic investigation of the negative food effect of modified release zolpidem. Eur J Pharm Sci. 2017;102:284–98.
Pepin X. The use of PBBM and biomarkers to provide detailed understanding of in vivo dissolution and absorption for Acalabrutinib University of Maryland, College Park, MD2019 [Available from: https://cersi.umd.edu/sites/cersi.umd.edu/files/Day%203%20-%206%20Xavier%20Pepin%20Final.pdf.
Weitschies W, Friedrich C, Wedemeyer RS, Schmidtmann M, Kosch O, Kinzig M, et al. Bioavailability of amoxicillin and clavulanic acid from extended release tablets depends on intragastric tablet deposition and gastric emptying. Eur J Pharm Biopharm. 2008;70(2):641–8.
Weitschies W, Wedemeyer RS, Kosch O, Fach K, Nagel S, Soderlind E, et al. Impact of the intragastric location of extended release tablets on food interactions. J Control Release. 2005;108(2–3):375–85.
Parrott N, Suarez-Sharp S, Kesisoglou F, Pathak SM, Good D, Wagner C, et al. Best Practices in the Development and Validation of Physiologically Based Biopharmaceutics Modeling. A Workshop Summary Report. Journal of Pharmaceutical Sciences. 2021;110:584–93.
Tsamandouras N, Rostami-Hodjegan A, Aarons L. Combining the ‘bottom up’ and ‘top down’ approaches in pharmacokinetic modelling: fitting PBPK models to observed clinical data. Br J Clin Pharmacol. 2013;79(1):48–55.
Pepin X, Huckle JE, Alluri RV, Basu S, Dodd S, Parrott N, et al. Understanding mechanisms of food effect and developing reliable PBPK models using a middle-out approach. AAPS J. 2021;23.
The International Consortium for Innovation and Quality in Pharmaceutical Development (IQ); https://iqconsortium.org/
Kuemmel C, Yang Y, Zhang X, Florian J, Zhu H, Tegenge M, et al. Consideration of a Credibility Assessment Framework in Model-Informed Drug Development: Potential Application to Physiologically-Based Pharmacokinetic Modeling and Simulation. CPT: Pharmacometrics & Systems Pharmacology. 2020;9(1):21–8.
Davit B, Chen M-L, Conner D, Haidar S, Kim S, Lee C, et al. Implementation of a Reference-Scaled Average Bioequivalence Approach for Highly Variable Generic Drug Products by the US Food and Drug Administration. AAPS J. 2012;14(4):915–24.
Mitra A, Suarez-Sharp S, Pepin XJH, Flanagan T, Zhao Y, Kotzagiorgis E, et al. Applications of Physiologically Based Biopharmaceutics Modeling (PBBM) to support Drug Product Quality: A Workshop Summary Report. J Pharm Sci. 2021;110:594–609.
Wong Chi H, Siah Kien WEI, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20(2):273–86.
Achour B, Al-Majdoub ZM, Grybos-Gajniak A, Lea K, Kilford P, Zhang M, et al. Liquid Biopsy Enables Quantification of the Abundance and Interindividual Variability of Hepatic Enzymes and Transporters. Clin Pharmacol Ther. 2021;109(1):222–32.
Polasek TM, Rostami-Hodjegan A. Virtual Twins: Understanding the Data Required for Model-Informed Precision Dosing. Clin Pharmacol Ther. 2020;107(4):742–5.
Darwich AS, Polasek TM, Aronson JK, Ogungbenro K, Wright DFB, Achour B, et al. Model-Informed Precision Dosing: Background, Requirements, Validation, Implementation, and Forward Trajectory of Individualizing Drug Therapy. Annu Rev Pharmacol Toxicol. 2021;61(1):225–45.
Ferreira A, Lapa R, Vale N. PBPK Modeling and Simulation and Therapeutic Drug Monitoring: Possible Ways for Antibiotic Dose Adjustment. Processes. 2021;9(11):2087.
Jereb R, Opara J, Bajc A, Petek B. Evaluating the Impact of Physiological Properties of the Gastrointestinal Tract On Drug In Vivo Performance Using Physiologically Based Biopharmaceutics Modeling and Virtual Clinical Trials. J Pharm Sci. 2021;110(8):3069–81.
Riethorst D, Mols R, Duchateau G, Tack J, Brouwers J, Augustijns P. Characterization of Human Duodenal Fluids in Fasted and Fed State Conditions. J Pharm Sci. 2016;105(2):673–81.
Guiastrennec BS DP, Hansen M, Bagger JL, Lund A, Rehfeld JF, Alskar O, Vilsbøll T, Knop FK, Bergstrand M. Mechanism‐Based Modeling of Gastric Emptying Rate and Gallbladder Emptying in Response to Caloric Intake. CPT: Pharmacomet Syst Pharmacol. 2016;5(12):692–700.
Rabbie SC, Flanagan T, Martin PD, Basit AW. Inter-subject variability in intestinal drug solubility. Int J Pharm. 2015;485(1–2):229–34.
Hofsäss MA, Dressman J. Evaluation of Differences in Dosage Form Performance of Generics Using BCS-Based Biowaiver Specifications and Biopharmaceutical Modeling-Case Examples Amoxicillin and Doxycycline. J Pharm Sci. 2020;109(8):2437–53.
Gisolfi CV, Summers RW, Lambert GP, Xia T. Effect of beverage osmolality on intestinal fluid absorption during exercise. Journal of applied physiology (Bethesda, Md : 1985). 1998;85(5):1941–8.
Lambert PGL, S., Welch R, Shi, X. Combined effects of glucose and fructose on fluid absorption from hypertonic carbohydrate-electrolyte beverages. Journal of Exercise Physiology. 2008;11(2):46–55.
Grimm M, Koziolek M, Saleh M, Schneider F, Garbacz G, Kühn J-P, et al. Gastric Emptying and Small Bowel Water Content after Administration of Grapefruit Juice Compared to Water and Isocaloric Solutions of Glucose and Fructose: A Four-Way Crossover MRI Pilot Study in Healthy Subjects. Mol Pharm. 2018;15(2):548–59.
Arbós P. Influence of the surface characteristics of PVM/MA nanoparticles on their bioadhesive properties. J Control Release. 2003;89(1):19–30.
Lipka E, Lee ID, Langguth P, Spahn-Langguth H, Mutschler E, Amidon GL. Celiprolol double-peak occurrence and gastric motility: nonlinear mixed effects modeling of bioavailability data obtained in dogs. J Pharmacokinet Biopharm. 1995;23(3):267–86.
Collins PJ, Houghton LA, Read NW, Horowitz M, Chatterton BE, Heddle R, et al. Role of the proximal and distal stomach in mixed solid and liquid meal emptying. Gut. 1991;32(6):615–9.
Anjiki H, Sanaka M, Kuyama Y. Dual effects of rabeprazole on solid-phase gastric emptying assessed by the 13C-octanoate breath test. Digestion. 2005;72(2–3):189–94.
Sager M, Jedamzik P, Merdivan S, Grimm M, Schneider F, Kromrey M-L, et al. Low dose caffeine as a salivary tracer for the determination of gastric water emptying in fed and fasted state: A MRI validation study. Eur J Pharm Biopharm. 2018;127:443–52.
Meyer JH, Dressman J, Fink A, Amidon G. Effect of size and density on canine gastric emptying of nondigestible solids. Gastroenterology. 1985;89(4):805–13.
Grimm M, Scholz E, Koziolek M, Kuhn JP, Weitschies W. Gastric Water Emptying under Fed State Clinical Trial Conditions Is as Fast as under Fasted Conditions. Mol Pharm. 2017;14(12):4262–71.
Locatelli I, Mrhar A, Bogataj M. Gastric Emptying of Pellets under Fasting Conditions: A Mathematical Model. Pharm Res. 2009;26(7):1607–17.
Alskar OB J, Røge RM, Knop FK, Karlsson MO, Vilsbøll T, Kjellsson MC. Semimechanistic model describing gastric emptying and glucose absorption in healthy subjects and patients with type 2 diabetes. J Clin Pharmacol. 2016;56(3):340–8.
Cucchiara S, Franzese A, Salvia G, Alfonsi L, Iula VD, Montisci A, et al. Gastric Emptying Delay and Gastric Electrical Derangement in IDDM. Diabetes Care. 1998;21(3):438–43.
Regårdh CG, Lundborg P, Persson BA. The effect of antacid, metoclopramide, and propantheline on the bioavailability of metoprolol and atenolol. Biopharm Drug Dispos. 1981;2(1):79–87.
Marathe PH, Wen Y, Norton J, Greene DS, Barbhaiya RH, Wilding IR. Effect of altered gastric emptying and gastrointestinal motility on metformin absorption. Br J Clin Pharmacol. 2000;50(4):325–32.
de Waal T, Rubbens J, Grimm M, Vandecaveye V, Tack J, Weitschies W, et al. Exploring the Effect of Esomeprazole on Gastric and Duodenal Fluid Volumes and Absorption of Ritonavir. Pharmaceutics. 2020;12(7).
Schulze JDR, Waddington WA, Ell PJ, Parsons GE, Coffin MD, Basit AW. Concentration-Dependent Effects of Polyethylene Glycol 400 on Gastrointestinal Transit and Drug Absorption. Pharm Res. 2003;20(12):1984–8.
Basit AW, Newton JM, Short MD, Waddington WA, Ell PJ, Lacey LF. The Effect of Polyethylene Glycol 400 on Gastrointestinal Transit: Implications for the Formulation of Poorly-Water Soluble Drugs. Pharm Res. 2001;18(8):1146–50.
Adkin D, Davis S, Sparrow R, Huckle P, Phillips A, Wilding I. The Effect of Different Concentrations of Mannitol in Solution on Small Intestinal Transit: Implications for Drug Absorption. Pharm Res. 1995;12(3):393–6.
Cvijić S, Parojčić J, Langguth P. Viscosity-mediated negative food effect on oral absorption of poorly-permeable drugs with an absorption window in the proximal intestine: In vitro experimental simulation and computational verification. Eur J Pharm Sci. 2014;61:40–53.
Radwan A, Amidon GL, Langguth P. Mechanistic investigation of food effect on disintegration and dissolution of BCS class III compound solid formulations: the importance of viscosity. Biopharm Drug Dispos. 2012;33(7):403–16.
Markl D, Zeitler JA. A Review of Disintegration Mechanisms and Measurement Techniques. Pharm Res. 2017;34(5):890–917.
Hörter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Deliv Rev. 1997;25:3–14.
Mitra A, Parrott N, Miller N, Lloyd R, Tistaert C, Heimbach T, et al. Prediction of pH-dependent drug-drug interactions for basic drugs using physiologically based biopharmaceutics modeling: industry case studies. J Pharm Sci. 2020;109(3):1380–94.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors have no conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anand, O., Pepin, X.J.H., Kolhatkar, V. et al. The Use of Physiologically Based Pharmacokinetic Analyses—in Biopharmaceutics Applications -Regulatory and Industry Perspectives. Pharm Res 39, 1681–1700 (2022). https://doi.org/10.1007/s11095-022-03280-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03280-4