Abstract
Purpose
Use of Flory–Huggins interaction parameter and contact angle values to predict the suitability of the drug-polymer system for the production and stability of nanosuspensions.
Material and Methods
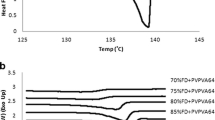
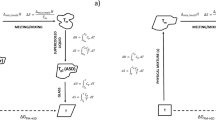
Melting point depression of the drug was measured using differential scanning calorimetry. Interaction parameter, χ, was calculated using the melting point depression data to elucidate the drug-polymer interaction strength to predict the suitability of the drug-polymer system for the production and stability of nanosuspensions. Contact angle of the drug films were measured with purified water and 0.1%w/w polymer solutions to predict polymer’s suitability for the production and stability of nanosuspension. Nanosuspensions were manufactured to validate the application of the melting point depression approach along with surface property information.
Results
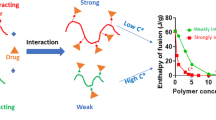
All three polymers, HPMC, Soluplus®, and poloxamer exhibited a negative interaction parameter with naproxen and budesonide. Higher negative interaction parameter values for the naproxen-polymer system indicated stronger drug-polymer interactions, while smaller negative interaction parameter values for the budesonide-polymer system indicated weaker drug-polymer interactions. Interaction parameter was not obtained for fenofibrate with HPMC and Soluplus®, and similarly, no interaction parameter was obtained for carvedilol with HPMC, most likely due to weaker drug-polymer interactions. All three polymers provided lower equilibrium contact angle values when compared to purified water, indicating an affinity for polymers.
Conclusions
Successful production and stability of several nanosuspensions were correlated with Flory–Huggins’s interaction parameter and contact angle values. In the absence of melting point depression, contact angle values can also be used predict the agglomeration tendencies as we have shown for this study.















Similar content being viewed by others
References
Kesisoglou F, Panmai S, Wu Y. Nanosizing–oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–44.
Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm. 2004;58(2):265–78.
Shegokar R, Muller RH. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm. 2010;399(1–2):129–39.
Keck CM, Müller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62(1):3–16.
Flory PJ. Principles of polymer chemistry. Ithaca,: Cornell University Press; 1953. 672 p. p.
Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm. 2006;321(1–2):1–11.
Müller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy: rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47(1):3–19.
Li X, Gu L, Xu Y, Wang Y. Preparation of fenofibrate nanosuspension and study of its pharmacokinetic behavior in rats. Drug Dev Ind Pharm. 2009;35(7):827–33.
Pardeike J, Strohmeier DM, Schrödl N, Voura C, Gruber M, Khinast JG, et al. Nanosuspensions as advanced printing ink for accurate dosing of poorly soluble drugs in personalized medicines. Int J Pharm. 2011;420(1):93–100.
Li M, Azad M, Dave R, Bilgili E. Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective. Pharmaceutics. 2016;8(2).
Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug-polymer miscibility and solubility. Pharm Res. 2006;23(10):2417–26.
Djuris J, Nikolakakis I, Ibric S, Djuric Z, Kachrimanis K. Preparation of carbamazepine-Soluplus solid dispersions by hot-melt extrusion, and prediction of drug-polymer miscibility by thermodynamic model fitting. Eur J Pharm Biopharm. 2013;84(1):228–37.
Altamimi MA, Neau SH. Use of the Flory-Huggins theory to predict the solubility of nifedipine and sulfamethoxazole in the triblock, graft copolymer Soluplus. Drug Dev Ind Pharm. 2016;42(3):446–55.
Altamimi MA, Neau SH. A study to identify the contribution of Soluplus® component homopolymers to the solubilization of nifedipine and sulfamethoxazole using the melting point depression method. Powder Technol. 2018;338:576–85.
Martin A. Physical Pharmacy: Lippincott Williams & Wilkins; 1993. p. 621.
Kumar S, Xu X, Gokhale R, Burgess DJ. Formulation parameters of crystalline nanosuspensions on spray drying processing: a DoE approach. Int J Pharm. 2014;464(1–2):34–45.
Müller R, Jacobs C. Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm. 2002;237(1–2):151–61.
Mishra PR, Al Shaal L, Müller RH, Keck CM. Production and characterization of Hesperetin nanosuspensions for dermal delivery. Int J Pharm. 2009;371(1–2):182–9.
Lin D, Huang Y. A thermal analysis method to predict the complete phase diagram of drug-polymer solid dispersions. Int J Pharm. 2010;399(1–2):109–15.
Zhao Y, Inbar P, Chokshi HP, Malick AW, Choi DS. Prediction of the thermal phase diagram of amorphous solid dispersions by Flory-Huggins theory. J Pharm Sci. 2011;100(8):3196–207.
Nishi T, Wang T. Melting point depression and kinetic effects of cooling on crystallization in poly (vinylidene fluoride)-poly (methyl methacrylate) mixtures. Macromolecules. 1975;8(6):909–15.
Tian Y, Booth J, Meehan E, Jones DS, Li S, Andrews GP. Construction of drug–polymer thermodynamic phase diagrams using Flory-Huggins interaction theory: identifying the relevance of temperature and drug weight fraction to phase separation within solid dispersions. Mol Pharm. 2013;10(1):236–48.
Marsac PJ, Li T, Taylor LS. Estimation of drug–polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2009;26(1):139.
Yue PF, Li Y, Wan J, Yang M, Zhu WF, Wang CH. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. Novel role of stabilizer/drug property. Int J Pharm. 2013;454(1):269–77.
Jamzad S, Fassihi R. Role of surfactant and pH on dissolution properties of fenofibrate and glipizide–a technical note. AAPS PharmSciTech. 2006;7(2):E33.
Hamed R, Awadallah A, Sunoqrot S, Tarawneh O, Nazzal S, AlBaraghthi T, et al. pH-Dependent Solubility and Dissolution Behavior of Carvedilol-Case Example of a Weakly Basic BCS Class II Drug. AAPS PharmSciTech. 2016;17(2):418–26.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172(3):1126–41.
Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63(6):456–69.
Gregory J, Barany S. Adsorption and flocculation by polymers and polymer mixtures. Adv Colloid Interface Sci. 2011;169(1):1–12.
Van Eerdenbrugh B, Froyen L, Van Humbeeck J, Martens JA, Augustijns P, Van den Mooter G. Drying of crystalline drug nanosuspensions-the importance of surface hydrophobicity on dissolution behavior upon redispersion. Eur J Pharm Sci. 2008;35(1–2):127–35.
Kayaert P, Anné M, Van den Mooter G. Bead layering as a process to stabilize nanosuspensions: influence of drug hydrophobicity on nanocrystal reagglomeration following in-vitro release from sugar beads. J Pharm Pharmacol. 2011;63(11):1446–53.
Monteiro A, Afolabi A, Bilgili E. Continuous production of drug nanoparticle suspensions via wet stirred media milling: a fresh look at the Rehbinder effect. Drug Dev Ind Pharm. 2013;39(2):266–83.
Sievens-Figueroa L, Bhakay A, Jerez-Rozo JI, Pandya N, Romañach RJ, Michniak-Kohn B, et al. Preparation and characterization of hydroxypropyl methyl cellulose films containing stable BCS Class II drug nanoparticles for pharmaceutical applications. Int J Pharm. 2012;423(2):496–508.
Van Eerdenbrugh B, Vermant J, Martens JA, Froyen L, Van Humbeeck J, Augustijns P, et al. A screening study of surface stabilization during the production of drug nanocrystals. J Pharm Sci. 2009;98(6):2091–103.
Cerdeira AM, Mazzotti M, Gander B. Formulation and drying of miconazole and itraconazole nanosuspensions. Int J Pharm. 2013;443(1–2):209–20.
Rasenack N, Hartenhauer H, Müller BW. Microcrystals for dissolution rate enhancement of poorly water-soluble drugs. Int J Pharm. 2003;254(2):137–45.
Verma S, Huey BD, Burgess DJ. Scanning probe microscopy method for nanosuspension stabilizer selection. Langmuir. 2009;25(21):12481–7.
Zimmermann A, Millqvist-Fureby A, Elema MR, Hansen T, Müllertz A, Hovgaard L. Adsorption of pharmaceutical excipients onto microcrystals of siramesine hydrochloride: Effects on physicochemical properties. Eur J Pharm Biopharm. 2009;71(1):109–16.
Rouchotas C, Cassidy OE, Rowley G. Comparison of surface modification and solid dispersion techniques for drug dissolution. Int J Pharm. 2000;195(1):1–6.
Verma S, Gokhale R, Burgess DJ. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int J Pharm. 2009;380(1):216–22.
Cerdeira AM, Mazzotti M, Gander B. Miconazole nanosuspensions: influence of formulation variables on particle size reduction and physical stability. Int J Pharm. 2010;396(1–2):210–8.
Azad M, Afolabi A, Bhakay A, Leonardi J, Dave R, Bilgili E. Enhanced physical stabilization of fenofibrate nanosuspensions via wet co-milling with a superdisintegrant and an adsorbing polymer. Eur J Pharm Biopharm. 2015;94:372–85.
Knieke C, Azad MA, Davé RN, Bilgili E. A study of the physical stability of wet media-milled fenofibrate suspensions using dynamic equilibrium curves. Chem Eng Res Des. 2013;91(7):1245–58.
Liversidge GG, Conzentino P. Drug particle size reduction for decreasing gastric irritancy and enhancing absorption of naproxen in rats. Int J Pharm. 1995;125(2):309–13.
Liversidge GG, Cundy KC. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs International Journal of Pharmaceutics. 1995;125(1):91–7.
Merisko-Liversidge E, Sarpotdar P, Bruno J, Hajj S, Wei L, Peltier N, et al. Formulation and antitumor activity evaluation of nanocrystalline suspensions of poorly soluble anticancer drugs. Pharm Res. 1996;13(2):272–8.
Grau MJ, Kayser O, Muller RH. Nanosuspensions of poorly soluble drugs–reproducibility of small scale production. Int J Pharm. 2000;196(2):155–9.
Zheng JY, Bosch HW. Sterile Filtration of NanoCrystal™ Drug Formulations. Drug Dev Ind Pharm. 1997;23(11):1087–93.
Lee J. Drug Nano- and Microparticles Processed into Solid Dosage Forms: Physical Properties. J Pharm Sci. 2003;92(10):2057–68.
Evertsson H, Nilsson S. Microviscosity in Clusters of Ethyl Hydroxyethyl Cellulose and Sodium Dodecyl Sulfate Formed in Dilute Aqueous Solutions As Determined with Fluorescence Probe Techniques. Macromolecules. 1997;30(8):2377–85.
Berglund KD, Przybycien TM, Tilton RD. Coadsorption of Sodium Dodecyl Sulfate with Hydrophobically Modified Nonionic Cellulose Polymers. 1 Role of Polymer Hydrophobic Modification Langmuir. 2003;19(7):2705–13.
Lee J, Lee S-J, Choi J-Y, Yoo JY, Ahn C-H. Amphiphilic amino acid copolymers as stabilizers for the preparation of nanocrystal dispersion. Eur J Pharm Sci. 2005;24(5):441–9.
Choi J-Y, Yoo JY, Kwak H-S, Uk Nam B, Lee J. Role of polymeric stabilizers for drug nanocrystal dispersions. Curr Appl Phys. 2005;5(5):472–4.
Acknowledgements
Thanks to BASF and Ashland for providing the polymer samples free of charge. The author thanks Dr. Shah and Dr. Desai for providing some of the drugs for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, R.K., Jonnalagadda, S. & Gupta, P.K. Use of Flory–Huggins Interaction Parameter and Contact Angle Values to Predict the Suitability of the Drug-Polymer System for the Production and Stability of Nanosuspensions. Pharm Res 39, 1001–1017 (2022). https://doi.org/10.1007/s11095-022-03269-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03269-z