Abstract
Purpose
The purpose of the present study was to investigate the dissolution profiles of cocrystals with cis–trans isomeric coformers. Previously, the carbamazepine (CBZ) cocrystals with even-carbon dicarboxylic acids showed higher supersaturation than those with odd-carbon ones, attributed to particle surface solution-mediated phase transformation (PS-SMPT) to CBZ dihydrate (CBZ DH). However, it has been unknown whether this odd–even pattern holds for cis–trans isomeric coformers.
Method
CBZ cocrystals with maleic acid (MLE) and fumaric acid (FUM) (CBZ-FUM anhydrate (CBZ-FUM AH) and monohydrate (CBZ-FUM H2O)) were employed as model cocrystals. Hydroxypropyl methylcellulose (HPMC), polyvinylpyrrolidone, and polyethylene glycol 6000 (PEG) were used as precipitation inhibitors. Dissolution tests were performed under a non-sink condition. Residual particles were analyzed by powder X-ray diffraction, differential scanning calorimetry, polarized light microscope, and scanning electron microscope.
Results
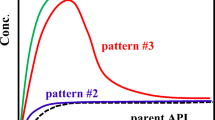
All cocrystals showed little supersaturation in the absence of a polymer. In 0.1% HPMC, CBZ-FUM AH showed significant supersaturation, whereas CBZ-MLE and CBZ-FUM H2O did not for the first two hours. HPMC reduced the initial dissolution rate of CBZ-MLE and CBZ-FUM H2O while inducing the highest supersaturation among the polymers after 96 h. The particle surface changed from a smooth plane to a striped pattern, but little or no CBZ DH was detected.
Conclusion
The cocrystals with cis–trans isomeric coformers showed different dissolution profiles. HPMC increased the dissolution rate of CBZ-FUM AH by inhibiting PS-SMPT but reduced the dissolution rate of CBZ-MLE and CBZ-FUM H2O without inducing PS-SMPT. The striped pattern was suggested to be due to surface etching rather than PS-SMPT.









Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Sugano K, Okazaki A, Sugimoto S, Tavornvipas S, Omura A, Mano T. Solubility and dissolution profile assessment in drug discovery. Drug Metab Pharmacokinet. 2007;22:225–54.
Lipinski C, et al. Poor aqueous solubility—an industry wide problem in drug discovery. Am Pharm Rev. 2002;5:82–5.
Ku MS, Dulin W. A biopharmaceutical classification-based Right-First-Time formulation approach to reduce human pharmacokinetic variability and project cycle time from First-In-Human to clinical Proof-Of-Concept. Pharmaceutical development and technology. Taylor \& Francis; 2012;17:285–302.
Wong SN, Chen YCS, Xuan B, Sun CC, Chow SF. Cocrystal Engineering of Pharmaceutical Solids: Therapeutic Potentials and Challenges. CrystEngComm: Royal Society of Chemistry; 2021.
Hisada N, Takano R, Takata N, Shiraki K, Ueto T, Tanida S, et al. Characterizing the dissolution profiles of supersaturable salts, cocrystals, and solvates to enhance in vivo oral absorption. European Journal of Pharmaceutics and Biopharmaceutics. Elsevier B.V.; 2016;103:192–9.
Thakuria R, Delori A, Jones W, Lipert MP, Roy L, Rodr\’\iguez-Hornedo N. Pharmaceutical cocrystals and poorly soluble drugs. International journal of pharmaceutics. Elsevier; 2013;453:101–25.
Bavishi DD, Borkhataria CH. Spring and parachute: How cocrystals enhance solubility. Prog Cryst Growth Charact Mater. 2016;62:1–8.
Wang Z-Z, Chen J-M, Lu T-B. Enhancing the hygroscopic stability of S-oxiracetam via pharmaceutical cocrystals. Crystal growth \& design. ACS Publications; 2012;12:4562–6.
Chattoraj S, Shi L, Sun CC. Understanding the relationship between crystal structure, plasticity and compaction behaviour of theophylline, methyl gallate, and their 1: 1 co-crystal. CrystEngComm Royal Society of Chemistry. 2010;12:2466–72.
Kuminek G, Cao F, da Rocha AB de O, Cardoso SG, Rodr\’\iguez-Hornedo N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Advanced drug delivery reviews. Elsevier; 2016;101:143–66.
Rahman Z, Agarabi C, Zidan AS, Khan SR, Khan MA. Physico-mechanical and stability evaluation of carbamazepine cocrystal with nicotinamide. Aaps Pharmscitech Springer. 2011;12:693–704.
Schultheiss N, Newman A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst Growth Des. 2009;9:2950–67.
Stanton MK, Bak A. Physicochemical properties of pharmaceutical co-crystals: A case study of ten AMG 517 co-crystals. Crystal Growth and Design. 2008
Aitipamula S, Banerjee R, Bansal AK, Biradha K, Cheney ML, Choudhury AR, et al. Polymorphs, salts, and cocrystals: what’s in a name? Crystal growth \& design. ACS Publications. 2012;12:2147–52.
Taylor LS, Braun DE, Steed JW. Crystals and Crystallization in Drug Delivery Design. ACS Publications; 2021.
Kataoka M, Minami K, Takagi T, Amidon GE, Yamashita S. In Vitro–In Vivo Correlation in Cocrystal Dissolution: Consideration of Drug Release Profiles Based on Coformer Dissolution and Absorption Behavior. Molecular Pharmaceutics: ACS Publications; 2021.
Yoshimura M, Miyake M, Kawato T, Bando M, Toda M, Kato Y, et al. Impact of the dissolution profile of the cilostazol cocrystal with supersaturation on the oral bioavailability. Crystal Growth \& Design. ACS Publications; 2017;17:550–7.
Huang Y, Kuminek G, Roy L, Cavanagh KL, Yin Q, Rodriguez-Hornedo N. Cocrystal Solubility Advantage Diagrams as a Means to Control Dissolution, Supersaturation, and Precipitation. Molecular pharmaceutics. 2019
Karimi-Jafari M, Padrela L, Walker GM, Croker DM. Creating cocrystals: a review of pharmaceutical cocrystal preparation routes and applications. Crystal Growth \& Design. ACS Publications; 2018;18:6370–87.
Childs SL, Kandi P, Lingireddy SR. Formulation of a danazol cocrystal with controlled supersaturation plays an essential role in improving bioavailability. Molecular Pharmaceutics. 2013
Greco K, Bogner R. Solution-mediated phase transformation: significance during dissolution and implications for bioavailability. Journal of pharmaceutical sciences Elsevier. 2012;101:2996–3018.
Greco K, Bogner R. Solution-mediated phase transformation: Significance during dissolution and implications for bioavailability. Journal of Pharmaceutical Sciences [Internet]. Elsevier Masson SAS; 2012;101:2996–3018. Available from: https://doi.org/10.1002/jps.23025
Yamashita H, Sun CC. Improving Dissolution Rate of Carbamazepine-Glutaric Acid Cocrystal Through Solubilization by Excess Coformer. Pharmaceutical Research. Pharmaceutical Research; 2018;35.
Yamashita H, Sun CC. Self-templating accelerates precipitation of carbamazepine dihydrate during the dissolution of a soluble carbamazepine cocrystal. CrystEngComm [Internet]. Royal Society of Chemistry; 2017;19:1156–9. Available from: https://doi.org/10.1039/C6CE02418A
Qiao N, Wang K, Schlindwein W, Davies A, Li M. In situ monitoring of carbamazepine-nicotinamide cocrystal intrinsic dissolution behaviour. European Journal of Pharmaceutics and Biopharmaceutics. 2013;
Yamashita H, Sun CC. Harvesting Potential Dissolution Advantages of Soluble Cocrystals by Depressing Precipitation Using the Common Coformer Effect. Cryst Growth Des. 2016;16:6719–21.
Omori M, Uekusa T, Oki J, Inoue D, Sugano K. Solution-mediated phase transformation at particle surface during cocrystal dissolution. Journal of Drug Delivery Science and Technology. Elsevier; 2020;56:101566.
Omori M, Watanabe T, Uekusa T, Oki J, Inoue D, Sugano K. Effects of Coformer and Polymer on Particle Surface Solution-Mediated Phase Transformation of Cocrystals in Aqueous Media. Mol Pharm. 2020;17:3825–36.
Shigemura M, Omori M, Sugano K. Polymeric precipitation inhibitor differently affects cocrystal surface and bulk solution phase transformations. Journal of Drug Delivery Science and Technology. Elsevier; 2021;103029.
Tao F, Bernasek SL. Understanding odd- even effects in organic self-assembled monolayers. Chemical reviews ACS Publications. 2007;107:1408–53.
Karki S, Friščić T, Jones W, Motherwell WDS. Screening for pharmaceutical cocrystal hydrates via neat and liquid-assisted grinding. Molecular pharmaceutics ACS Publications. 2007;4:347–54.
Perumalla SR, Wang C, Guo Y, Shi L, Sun CC. Robust bulk preparation and characterization of sulfamethazine and saccharine salt and cocrystal polymorphs. CrystEngComm Royal Society of Chemistry. 2019;21:2089–96.
Porter Iii WW, Elie SC, Matzger AJ. Polymorphism in carbamazepine cocrystals. Crystal Growth and Design ACS Publications. 2008;8:14–6.
Trask A V, van de Streek J, Motherwell WDS, Jones W. Achieving polymorphic and stoichiometric diversity in cocrystal formation: Importance of solid-state grinding, powder X-ray structure determination, and seeding. Crystal growth \& design. ACS Publications; 2005;5:2233–41.
Sugano K. Fraction of a dose absorbed estimation for structurally diverse low solubility compounds. International Journal of Pharmaceutics. 2011;405.
Childs SL, Wood PA, Rodríguez-Hornedo N, Reddy LS, Hardcastle KI. Analysis of 50 crystal structures containing carbamazepine using the materials module of mercury CSD. Crystal Growth and Design ACS Publications. 2009;9:1869–88.
Childs SL, Rodríguez-Hornedo N, Reddy LS, Jayasankar A, Maheshwari C, McCausland L, et al. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. CrystEngComm. 2008;10:856–64.
Fleischman SG, Kuduva SS, McMahon JA, Moulton B, Bailey Walsh RD, Rodr\’\iguez-Hornedo N, et al. Crystal engineering of the composition of pharmaceutical phases: multiple-component crystalline solids involving carbamazepine. Crystal Growth \& Design. ACS Publications; 2003;3:909–19.
Liu X, Osawa T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochemical and biophysical research communications Elsevier. 2007;357:187–93.
Dugave C, Demange L. Cis- trans isomerization of organic molecules and biomolecules: implications and applications. Chemical reviews ACS Publications. 2003;103:2475–532.
Saftić D, Studzińska M, Paradowska E, Piantanida I, Baranović G, Białek-Pietras M, et al. Comparative study of the effects of ortho-, meta-and para-carboranes (C2B10H12) on the physicochemical properties, cytotoxicity and antiviral activity of uridine and 2′-deoxyuridine boron cluster conjugates. Bioorganic chemistry. Elsevier; 2020;94:103466.
Wu W, Wang Y, Löbmann K, Grohganz H, Rades T. Transformations between co-amorphous and co-crystal systems and their influence on the formation and physical stability of co-amorphous systems. Molecular pharmaceutics ACS Publications. 2019;16:1294–304.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods. Nature Publishing Group; 2012;9:671.
Abd Rahim S, Tan CC, Ramle NA. Carbamazepine-fumaric acid co-crystal screening using solution based method. MATEC Web of Conferences. 2016. p. 3003.
Uekusa T, Sugano K. Precipitation behavior of pioglitazone on the particle surface of hydrochloride salt in biorelevant media. Journal of Pharmaceutical and Biomedical Analysis. Elsevier B.V.; 2018;161:45–50.
Good DJ, Naír RH. Solubility advantage of pharmaceutical cocrystals. Crystal Growth and Design. 2009;
Hickey MB, Peterson ML, Scoppettuolo LA, Morrisette SL, Vetter A, Guzmán H, et al. Performance comparison of a co-crystal of carbamazepine with marketed product. European journal of pharmaceutics and biopharmaceutics. Elsevier; 2007;67:112–9.
Grimm M, Koziolek M, Saleh M, Schneider F, Garbacz G, Kühn JP, et al. Gastric Emptying and Small Bowel Water Content after Administration of Grapefruit Juice Compared to Water and Isocaloric Solutions of Glucose and Fructose: A Four-Way Crossover MRI Pilot Study in Healthy Subjects. Mol Pharm. 2018;15:548–59.
Kuldipkumar A, Tan YTF, Goldstein M, Nagasaki Y, Zhang GGZ, Kwon GS. Amphiphilic block copolymer as a crystal habit modifier. Crystal growth \& design. ACS Publications; 2005;5:1781–5.
Lang M, Grzesiak AL, Matzger AJ. The use of polymer heteronuclei for crystalline polymorph selection. Journal of the American Chemical Society. ACS Publications; 2002;124:14834–5.
Pudipeddi M, Serajuddin ATM. Trends in solubility of polymorphs. J Pharm Sci. 2005;94:929–39.
Alhalaweh A, Roy L, Rodríguez-Hornedo N, Velaga SP. PH-dependent solubility of indomethacin-saccharin and carbamazepine- saccharin cocrystals in aqueous media. Mol Pharm. 2012;9:2605–12.
Uekusa T, Avdeef A, Sugano K. Is equilibrium slurry pH a good surrogate for solid surface pH during drug dissolution? European Journal of Pharmaceutical Sciences. Elsevier; 2022;168:106037.
Omori M, Watanabe T, Uekusa T, Oki J, Inoue D, Sugano K. Effects of Coformer and Polymer on Particle Surface Solution-Mediated Phase Transformation of Cocrystals in Aqueous Media. Molecular Pharmaceutics. 2020;
Omori M, Sugano K. Solution-Mediated Phase Transformation on Crystal Facets of Carbamazepine--Saccharin Cocrystals. Crystal Growth \& Design. ACS Publications; 2021;
Vasil’Chenko MA, Shakhtshneider TP, Naumov DY, Boldyrev V V. Topochemistry of the initial stages of the dissolution of single crystals of acetaminophen. Journal of pharmaceutical sciences. Elsevier; 1996;85:929–34.
Tian F, Sandler N, Gordon KC, McGoverin CM, Reay A, Strachan CJ, et al. Visualizing the conversion of carbamazepine in aqueous suspension with and without the presence of excipients: a single crystal study using SEM and Raman microscopy. European journal of pharmaceutics and biopharmaceutics. Elsevier; 2006;64:326–35.
Wen H, Li T, Morris KR, Park K. How solvents affect acetaminophen etching pattern formation: Interaction between solvent and acetaminophen at the solid/liquid interface. The Journal of Physical Chemistry B. ACS Publications; 2004;108:2270–8.
Kirubakaran P, Wang K, Rosbottom I, Cross RBM, Li M. Understanding the effects of a polymer on the surface dissolution of pharmaceutical cocrystals using combined experimental and molecular dynamics simulation approaches. Molecular Pharmaceutics: ACS Publications; 2019.
Wen H, Morris KR, Park K. Synergic effects of polymeric additives on dissolution and crystallization of acetaminophen. Pharm Res. 2008;25:349–58.
Rahim SA, Hammond RB, Sheikh AY, Roberts KJ. A comparative assessment of the influence of different crystallization screening methodologies on the solid forms of carbamazepine co-crystals. CrystEngComm. 2013;15.
Acknowledgements and Disclosures
The Author(s) declare(s) that they have no conflicts of interest to disclose.
Funding
This research was partly supported by AMED (Grant Number: JP17ak0101074).
Author information
Authors and Affiliations
Contributions
Maaya Omori: Data acquisition, Data curation, Study design, Writing.
Hibiki Yamamoto: Data acquisition, Data curation.
Fumiya Matsui: Data acquisition, Data curation.
Kiyohiko Sugano: Study design, Supervision, Funding acquisition, Writing.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Omori, M., Yamamoto, H., Matsui, F. et al. Dissolution Profiles of Carbamazepine Cocrystals with Cis–Trans Isomeric Coformers. Pharm Res 40, 579–591 (2023). https://doi.org/10.1007/s11095-022-03209-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03209-x