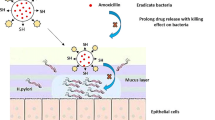
Abstract
Purpose
This study was undertaken to develop novel mucoadhesive formulations of clofazimine (CFZ), a drug candidate for the treatment of cryptosporidiosis, with the aim of strategic delivery to the small intestine, the main site of the disease parasites.
Methods
CFZ-loaded nanoparticles (nCFZ) coated with non-biodegradable anionic polymer (nCFZ/A) and biodegradable anionic protein complex (nCFZ/dA) were prepared by Flash NanoPrecipitation (FNP) and evaluated for their physicochemical and biopharmaceutical properties.
Results
The mean diameters of nCFZ/A and nCFZ/dA were ca. 90 and 240 nm, respectively, and they showed narrow size distributions and negative ζ-potentials. Both formulations showed higher solubility of CFZ in aqueous solution than crystalline CFZ. Despite their improved dispersion behaviors, both formulations exhibited significantly lower diffusiveness than crystalline CFZ in a diffusion test using artificial mucus (AM). Quartz crystal microbalance analysis showed that both formulations clearly interacted with mucin, which appeared to be responsible for their reduced diffusiveness in AM. These results suggest the potent mucoadhesion of nCFZ/A and nCFZ/dA. After the oral administration of CFZ samples (10 mg-CFZ/kg) to rats, nCFZ/dA and nCFZ/A exhibited a prolongation in Tmax by 2 and >9 h, respectively, compared with crystalline CFZ. At 24 h after oral doses of nCFZ/A and nCFZ/dA with mucoadhesion, there were marked increases in the intestinal CFZ concentration (4–7 fold) compared with Lamprene®, a commercial CFZ product, indicating enhanced CFZ exposure in the small intestine.
Conclusion
The use of FNP may produce mucoadhesive CFZ formulations with improved intestinal exposure, possibly offering enhanced anti-cryptosporidium therapy.




Similar content being viewed by others
References
Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric Multicenter study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22.
Manabe YC, Clark DP, Moore RD, Lumadue JA, Dahlman HR, Belitsos PC, Chaisson RE, Sears CL. Cryptosporidiosis in patients with AIDS: correlates of disease and survival. Clin Infect Dis. 1998;27(3):536–42.
Bouzid M, Hunter PR, Chalmers RM, Tyler KM. Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev. 2013;26(1):115–34.
Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360(9343):1375–80.
Amadi B, Mwiya M, Sianongo S, Payne L, Watuka A, Katubulushi M, Kelly P. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC Infect Dis. 2009;9:195.
Manjunatha UH, Vinayak S, Zambriski JA, Chao AT, Sy T, Noble CG, Bonamy GMC, Kondreddi RR, Zou B, Gedeck P, Brooks CF, Herbert GT, Sateriale A, Tandel J, Noh S, Lakshminarayana SB, Lim SH, Goodman LB, Bodenreider C, et al. A Cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis. Nature. 2017;546(7658):376–80.
Hewitt RG, Yiannoutsos CT, Higgs ES, Carey JT, Geiseler PJ, Soave R, Rosenberg R, Vazquez GJ, Wheat LJ, Fass RJ, Antoninievic Z, Walawander AL, Flanigan TP, Bender JF. Paromomycin: no more effective than placebo for treatment of cryptosporidiosis in patients with advanced human immunodeficiency virus infection. AIDS clinical trial group. Clin Infect Dis. 2000;31(4):1084–92.
Love MS, Beasley FC, Jumani RS, Wright TM, Chatterjee AK, Huston CD, Schultz PG, McNamara CW. A high-throughput phenotypic screen identifies clofazimine as a potential treatment for cryptosporidiosis. PLoS Negl Trop Dis. 2017;11(2):e0005373.
Baik J, Stringer KA, Mane G, Rosania GR. Multiscale distribution and bioaccumulation analysis of clofazimine reveals a massive immune system-mediated xenobiotic sequestration response. Antimicrob Agents Chemother. 2013;57(3):1218–30.
Netsomboon K, Bernkop-Schnurch A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur J Pharm Biopharm. 2016;98:76–89.
Takeuchi H, Matsui Y, Yamamoto H, Kawashima Y. Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J Control Release. 2003;86(2–3):235–42.
Suwannateep N, Banlunara W, Wanichwecharungruang SP, Chiablaem K, Lirdprapamongkol K, Svasti J. Mucoadhesive curcumin nanospheres: biological activity, adhesion to stomach mucosa and release of curcumin into the circulation. J Control Release. 2011;151(2):176–82.
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: in vivo performances. J Control Release. 2012;160(3):418–30.
Zhang Y, Feng J, McManus SA, Lu HD, Ristroph KD, Cho EJ, Dobrijevic EL, Chan HK, Prud'homme RK. Design and solidification of fast-releasing Clofazimine nanoparticles for treatment of cryptosporidiosis. Mol Pharm. 2017;14(10):3480–8.
Johnson BK, Prud'homme RK. Flash NanoPrecipitation of organic actives and block copolymers using a confined impinging jets mixer. Aust J Chem. 2003;56(10):1021–4.
Liu Y, Cheng CY, Liu Y, Prud'homme RK, Fox RO. Mixing in a multi-inlet vortex mixer (MIVM) for flash nano-precipitation. Chem Eng Sci. 2008;63(11):2829–42.
Markwalter CE, Pagels RF, Wilson BK, Ristroph KD, Prud'homme RK. Flash NanoPrecipitation for the Encapsulation of Hydrophobic and Hydrophilic Compounds in Polymeric Nanoparticles. J Vis Exp. 2019(143).
Weissmueller NT, Lu HD, Hurley A, Prud'homme RK. Nanocarriers from GRAS Zein proteins to encapsulate hydrophobic actives. Biomacromolecules. 2016;17(11):3828–37.
Craparo EF, Porsio B, Sardo C, Giammona G, Cavallaro G. Pegylated Polyaspartamide-Polylactide-based nanoparticles penetrating cystic fibrosis artificial mucus. Biomacromolecules. 2016;17(3):767–77.
Sato H, Kaneko Y, Yamada K, Ristroph KD, Lu HD, Seto Y, Chan HK, Prud'homme RK, Onoue S. Polymeric Nanocarriers with mucus-diffusive and mucus-adhesive properties to control pharmacokinetic behavior of orally dosed cyclosporine a. J Pharm Sci. 2020;109(2):1079–85.
Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81(4):1930–7.
Ponchel G, Montisci MJ, Dembri A, Durrer C, Duchene D. Mucoadhesion of colloidal particulate systems in the gastro-intestinal tract. Eur J Pharm Biopharm. 1997;44(1):25–31.
Leal J, Smyth HDC, Ghosh D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm. 2017;532(1):555–72.
Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A. 2007;104(5):1482–7.
Feng J, Zhang Y, McManus SA, Ristroph KD, Lu HD, Gong K, White CE, Prud’homme RK. Rapid recovery of clofazimine-loaded nanoparticles with long-term storage stability as anti-cryptosporidium therapy. ACS applied nano materials. 2018;1(5):2184–94.
Jinno J, Kamada N, Miyake M, Yamada K, Mukai T, Odomi M, Toguchi H, Liversidge GG, Higaki K, Kimura T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J Control Release. 2006;111(1–2):56–64.
Chayed S, Winnik FM. In vitro evaluation of the mucoadhesive properties of polysaccharide-based nanoparticulate oral drug delivery systems. Eur J Pharm Biopharm. 2007;65(3):363–70.
Yawalkar SJ, Vischer W. Lamprene (Clofazimine) in Leprosy. Leprosy Rev. 1979;50(2):135–44.
Horter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Deliv Rev. 2001;46(1–3):75–87.
Funding
This work was financially supported by JSPS KAKENHI [a Grant-in-Aid for Scientific Research (C) (No. 20 K07158: S. Onoue, No. 20 K07180: H. Sato)]. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. #DGE-1656466 awarded to KDR.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamada, K., Ristroph, K.D., Kaneko, Y. et al. Clofazimine-Loaded Mucoadhesive Nanoparticles Prepared by Flash Nanoprecipitation for Strategic Intestinal Delivery. Pharm Res 38, 2109–2118 (2021). https://doi.org/10.1007/s11095-021-03144-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03144-3