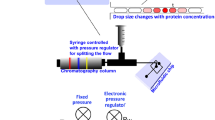
Abstract
Purpose
Measurement of the viscosity of concentrated protein solutions is vital for the manufacture and delivery of protein therapeutics. Conventional methods for viscosity measurements require large solution volumes, creating a severe limitation during the early stage of protein development. The goal of this work is to develop a robust technique that requires minimal sample.
Methods
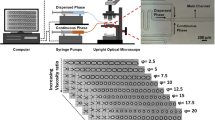
In this work, a droplet-based microfluidic device is developed to quantify the viscosity of protein solutions while concentrating in micrometer-scale droplets. The technique requires only microliters of sample. The corresponding viscosity is characterized by multiple particle tracking microrheology (MPT).
Results
We show that the viscosities quantified in the microfluidic device are consistent with macroscopic results measured by a conventional rheometer for poly(ethylene) glycol (PEG) solutions. The technique was further applied to quantify viscosities of well-studied lysozyme and bovine serum albumin (BSA) solutions. Comparison to both macroscopic measurements and models (Krieger-Dougherty model) demonstrate the validity of the approach.
Conclusion
The droplet-based microfluidic device provides accurate quantitative values of viscosity over a range of concentrations for protein solutions with small sample volumes (~ μL) and high compositional resolution. This device will be extended to study the effect of different excipients and other additives on the viscosity of protein solutions.
Graphical abstract






Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MPT:
-
Multiple particle tracking microrheology
- PEG:
-
Poly(ethylene) glycol
- BSA:
-
Bovine Serum Albumin
- MSD:
-
Mean-squared displacement
- PDMS:
-
Polydimethylsiloxane
References
Lagassé HD, Alexaki A, Simhadri VL, Katagiri NH, Jankowski W, Sauna ZE, Kimchi-Sarfaty C. Recent advances in (therapeutic protein) drug development. F1000Research. 2017;6 (F1000 Faculty Rev):113. https://doi.org/10.12688/f1000research.9970.1
Du W, Klibanov AM. Hydrophobic salts markedly diminish viscosity of concentrated protein solutions. Biotechnol Bioeng. 2011;108(3):632–6. https://doi.org/10.1002/bit.22983.
Inoue N, Takai E, Arakawa T, Shiraki K. Arginine and lysine reduce the high viscosity of serum albumin solutions for pharmaceutical injection. J Biosci Bioeng. 2014;117(5):539–43. https://doi.org/10.1016/j.jbiosc.2013.10.016.
Shire SJ, Shahrokh Z, Liu JU. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93(6):1390–402. https://doi.org/10.1002/jps.20079.
Harris RJ, Shire SJ, Winter C. Commercial manufacturing scale formulation and analytical characterization of therapeutic recombinant antibodies. Drug Dev Res. 2004;61(3):137–54. https://doi.org/10.1002/ddr.10344.
Berteau C, Filipe-Santos O, Wang T, Rojas HE, Granger C, Schwarzenbach F. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Medical Devices (Auckland, NZ). 2015;8:473. https://doi.org/10.2147/MDER.S91019.
Liu J, Nguyen MD, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci. 2006;95(1):234–5. https://doi.org/10.1002/jps.20347.
Johnson HR, Lenhoff AM. Characterization and suitability of therapeutic antibody dense phases for subcutaneous delivery. Mol Pharm. 2013;10(10):3582–91. https://doi.org/10.1021/mp400006g.
Daugherty AL, Mrsny RJ. Formulation and delivery issues for monoclonal antibody therapeutics. Adv Drug Deliv Rev. 2006;58(5–6):686–706. https://doi.org/10.1016/j.addr.2006.03.011.
Braun A, Kwee L, Labow MA, Alsenz J. Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFN-α) in normal and transgenic mice. Pharm Res. 1997;14(10):1472–8. https://doi.org/10.1023/A:1012193326789.
Koren E, Zuckerman LA, Mire-Sluis AR. Immune responses to therapeutic proteins in humans-clinical significance, assessment and prediction. Curr Pharm Biotechnol. 2002;3(4):349–60. https://doi.org/10.2174/1389201023378175.
Cleland JL, Powell MF, Shire SJ. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit Rev Ther Drug Carrier Syst. 1993;10(4):307–77.
He F, Woods CE, Litowski JR, Roschen LA, Gadgil HS, Razinkov VI, Kerwin BA. Effect of sugar molecules on the viscosity of high concentration monoclonal antibody solutions. Pharm Res. 2011;28(7):1552–60. https://doi.org/10.1007/s11095-011-0388-7.
Inoue N, Takai E, Arakawa T, Shiraki K. Specific decrease in solution viscosity of antibodies by arginine for therapeutic formulations. Mol Pharm. 2014;11(6):1889–96. https://doi.org/10.1021/mp5000218.
Wang S, Zhang N, Hu T, Dai W, Feng X, Zhang X, Qian F. Viscosity-lowering effect of amino acids and salts on highly concentrated solutions of two IgG1 monoclonal antibodies. Mol Pharm. 2015;12(12):4478–87. https://doi.org/10.1021/acs.molpharmaceut.5b00643.
Kanai S, Liu JU, Patapoff TW, Shire SJ. Reversible self-association of a concentrated monoclonal antibody solution mediated by Fab-Fab interaction that impacts solution viscosity. J Pharm Sci. 2008;97(10):4219–27. https://doi.org/10.1002/jps.21322.
Yadav S, Shire SJ, Kalonia DS. Viscosity analysis of high concentration bovine serum albumin aqueous solutions. Pharm Res. 2011;28(8):1973–83. https://doi.org/10.1007/s11095-011-0424-7.
Benoit SM, Afizah MN, Ruttarattanamongkol K, Rizvi SS. Effect of pH and temperature on the viscosity of texturized and commercial whey protein dispersions. Int J Food Prop. 2013;16(2):322–30. https://doi.org/10.1080/10942912.2011.552015.
Sharma V, Jaishankar A, Wang YC, McKinley GH. Rheology of globular proteins: apparent yield stress, high shear rate viscosity and interfacial viscoelasticity of bovine serum albumin solutions. Soft Matter. 2011;7(11):5150–60. https://doi.org/10.1039/C0SM01312A.
Castellanos MM, Pathak JA, Colby RH. Both protein adsorption and aggregation contribute to shear yielding and viscosity increase in protein solutions. Soft Matter. 2014;10(1):122–31. https://doi.org/10.1039/C3SM51994E.
Josephson LL, Furst EM, Galush WJ. Particle tracking microrheology of protein solutions. J Rheol. 2016;60(4):531–40. https://doi.org/10.1122/1.4948427.
Kim S, Kim KC, Yeom E. Microfluidic method for measuring viscosity using images from smartphone. Opt Lasers Eng. 2018;104:237–43. https://doi.org/10.1016/j.optlaseng.2017.05.016.
Wang G, Tan C, Li F. A contact resonance viscometer based on the electromechanical impedance of a piezoelectric cantilever. Sens Actuators, A. 2017;267:401–8. https://doi.org/10.1016/j.sna.2017.10.041.
Foffi G, Savin G, Bucciarelli S, Dorsaz N, Thurston GM, Stradner A, Schurtenberger P. Hard sphere-like glass transition in eye lens α-crystallin solutions. Proc Natl Acad Sci. 2014;111(47):16748–53. https://doi.org/10.1073/pnas.1406990111.
Heinen M, Zanini F, Roosen-Runge F, Fedunová D, Zhang F, Hennig M, Seydel T, Schweins R, Sztucki M, Antalík M, Schreiber F. Viscosity and diffusion: crowding and salt effects in protein solutions. Soft Matter. 2012;8(5):1404–19. https://doi.org/10.1039/C1SM06242E.
Sarangapani PS, Hudson SD, Migler KB, Pathak JA. The limitations of an exclusively colloidal view of protein solution hydrodynamics and rheology. Biophys J . 2013;105(10):2418–26. https://doi.org/10.1016/j.bpj.2013.10.012.
Bleier BJ, Anna SL, Walker LM. Microfluidic droplet-based tool to determine phase behavior of a fluid system with high composition resolution. J Phys Chem B. 2018;122(14):4067–76. https://doi.org/10.1021/acs.jpcb.8b01013.
Xia Y, Whitesides GM. Soft lithography. Annu Rev Mater Sci. 1998;28(1):153–84. https://doi.org/10.1146/annurev.matsci.28.1.153.
McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJ, Whitesides GM. Fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophoresis: Int J. 2000;21(1):27–40. https://doi.org/10.1002/(SICI)1522-2683(20000101)21:1%3c27::AID-ELPS27%3e3.0.CO;2-C.
Bleier BJ. Droplet-Based Approaches to Probe Complex Behavior in Colloidal Fluids with High Composition Resolution (No. 10816201). 2018; (Doctoral dissertation, Carnegie Mellon University). Retrieved from https://www.proquest.com/dissertations-theses/droplet-based-approaches-probecomplex-behavior/docview/2048167241/se-2?accountid=9902
Vuong SM. A microfluidic platform for the control and analysis of phase transitions in concentrating droplets (No. 3690561). 2014; (Doctoral dissertation, Carnegie Mellon University). Retrieved from https://www.proquest.com/dissertations-theses/microfluidic-platform-controlanalysis-phase/docview/1665305117/se-2?accountid=9902
Savin T, Doyle PS. Static and dynamic errors in particle tracking microrheology. Biophys J . 2005;88(1):623–38. https://doi.org/10.1529/biophysj.104.042457.
Crocker JC, Grier DG. Methods of digital video microscopy for colloidal studies. J Colloid Interface Sci. 1996;179(1):298–310. https://doi.org/10.1006/jcis.1996.0217.
Wehrman MD, Leduc A, Callahan HE, Mazzeo MS, Schumm M, Schultz KM. Rheological properties and structure of step-and chain-growth gels concentrated above the overlap concentration. AIChE J. 2018;64(8):3168–76. https://doi.org/10.1002/aic.16062.
Breedveld V, Pine DJ. Microrheology as a tool for high-throughput screening. J Mater Sci. 2003;38(22):4461–70. https://doi.org/10.1023/A:1027321232318.
Wehrman MD, Lindberg S, Schultz KM. Multiple particle tracking microrheology measured using bi-disperse probe diameters. Soft Matter. 2018;14(28):5811–20. https://doi.org/10.1039/C8SM01098F.
Swan JW, Brady JF. Particle motion between parallel walls: Hydrodynamics and simulation. Phys Fluids. 2010;22(10):103301. https://doi.org/10.1063/1.348774838.
McGlynn JA, Wu N, Schultz KM. Multiple particle tracking microrheological characterization: Fundamentals, emerging techniques and applications. J Appl Phys. 2020;127(20):201101. https://doi.org/10.1063/5.0006122.
Huggins ML. The viscosity of dilute solutions of long-chain molecules. IV. Dependence on concentration. J Am Chem Soc. 1942;64(11):2716–8.
Back DM, Schmitt RL. Ethylene oxide polymers. Kirk-Othmer Encycl Chem Technol. 2000. https://doi.org/10.1002/0471238961.
Ziębacz N, Wieczorek SA, Kalwarczyk T, Fiałkowski M, Hołyst R. Crossover regime for the diffusion of nanoparticles in polyethylene glycol solutions: influence of the depletion layer. Soft Matter. 2011;7(16):7181–6. https://doi.org/10.1039/C0SM01357A.
Krieger IM, Dougherty TJ. A mechanism for non-Newtonian flow in suspensions of rigid spheres. Transactions of the Society of Rheology. 1959;3(1):137–52. https://doi.org/10.1122/1.548848.
Quemada D, Berli C. Energy of interaction in colloids and its implications in rheological modeling. Adv Coll Interface Sci. 2002;98(1):51–85. https://doi.org/10.1016/S0001-8686(01)00093-8.
Donev A, Cisse I, Sachs D, Variano EA, Stillinger FH, Connelly R, Torquato S, Chaikin PM. Improving the density of jammed disordered packings using ellipsoids. Science. 2004 Feb;303(5660):990–3. https://doi.org/10.1126/science.1093010.
Zhang Z, Liu Y. Recent progresses of understanding the viscosity of concentrated protein solutions. Curr Opin Chem Eng. 2017;16:48–55. https://doi.org/10.1016/j.coche.2017.04.001.
Liu J, Thompson ZJ, Sue HJ, Bates FS, Hillmyer MA, Dettloff M, Jacob G, Verghese N, Pham H. Toughening of epoxies with block copolymer micelles of wormlike morphology. Macromolecules. 2010;43(17):7238–43. https://doi.org/10.1021/ma902471g.
Godfrin PD, Hudson SD, Hong K, Porcar L, Falus P, Wagner NJ, Liu Y. Short-time glassy dynamics in viscous protein solutions with competing interactions. Phys Rev Lett. 2015;115(22): 228302. https://doi.org/10.1103/PhysRevLett.115.228302.
Beverung CJ, Radke CJ, Blanch HW. Protein adsorption at the oil/water interface: characterization of adsorption kinetics by dynamic interfacial tension measurements. Biophys Chem. 1999;81(1):59–80. https://doi.org/10.1016/S0301-4622(99)00082-4.
T S, S D, . A New Methodology for Studying Protein Adsorption at Oil-Water Interfaces. J Colloid Interface Sci. 1998;206:407–15.
Garting T, Stradner A. Synthesis and application of PEGylated tracer particles for measuring protein solution viscosities using Dynamic Light Scattering-based microrheology. Colloids Surf, B. 2019;181:516–23. https://doi.org/10.1016/j.colsurfb.2019.05.059.
Furst EM, Squires TM. Microrheology. Oxford University Press; 2017.
Acknowledgements
The authors would like to acknowledge Dr. Junchi Ma for bulk rheology measurements of 100 kg/mol PEG solutions. Funding for this work is provided by department of Chemical Engineering, Carnegie Mellon University. The authors report no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, D., Daviran, M., Schultz, K.M. et al. Droplet-Based Microfluidic Tool to Quantify Viscosity of Concentrating Protein Solutions. Pharm Res 38, 1765–1775 (2021). https://doi.org/10.1007/s11095-021-03106-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03106-9