Abstract
Purpose
Skin and soft tissue infections are increasingly prevalent and often complicated by potentially fatal therapeutic hurdles, such as poor drug perfusion and antibiotic resistance. Delivery vehicles capable of versatile loading may improve local bioavailability and minimize systemic toxicities yet such vehicles are not clinically available. Therefore, we aimed to expand upon the use of glutathione-conjugated poly(ethylene glycol) GSH-PEG hydrogels beyond protein delivery and evaluate the ability to deliver traditional therapeutic molecules.
Methods
PEG and GSH-PEG hydrogels were prepared using ultraviolet light (UV)-polymerization. Hydrogel loading and release of selected drug candidates was examined using UV-visible spectrometry. Therapeutic molecules and GST-fusion protein loading was examined using UV-visible and fluorescent spectrometry. Efficacy of released meropenem was assessed against meropenem-sensitive and -resistant P. aeruginosa in an agar diffusion bioassay.
Results
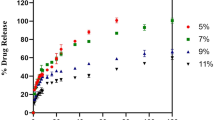
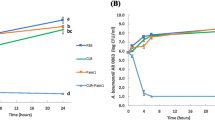
For all tested agents, GSH-PEG hydrogels demonstrated time-dependent loading whereas PEG hydrogels did not. GSH-PEG hydrogels released meropenem over 24 h. Co-loading of biologic and traditional therapeutics into a single vehicle was successfully demonstrated. Meropenem-loaded GSH-PEG hydrogels inhibited the growth of meropenem-sensitive and resistant P. aeruginosa isolates.
Conclusion
GSH ligands within GSH-PEG hydrogels allow loading and effective delivery of charged therapeutic agents, in addition to biologic therapeutics.
Graphical abstract











Similar content being viewed by others
Notes
No correction for the GSH consumption of PEGDA end groups was used similar to previous publications (23).
Abbreviations
- AR:
-
Antibiotic resistances
- CAMHB:
-
Cation-adjusted Mueller-Hinton cation-adjusted Mueller-Hinton broth
- C d :
-
Donor cell drug concentration
- CDC:
-
Center for Disease Control and Prevention
- CLSI:
-
Clinical and Laboratory Standards Institute
- C n :
-
Characteristic ratio
- D i :
-
Diffusion coefficient
- GSH:
-
Glutathione
- GSH-PEG:
-
Glutathione-conjugated polyethylene glycol
- GST:
-
Glutathione-S-transferase
- I :
-
Ionic strength
- IMF:
-
Intermolecular force
- j ss :
-
Flux of drug through membrane at steady state
- K i :
-
Partition coefficient
- l :
-
Bond length along the polymer backbone
- L :
-
Membrane thickness
- LMS:
-
Low molecular weight salt
- m 0 :
-
Mass of the dried hydrogel
- M c :
-
Molecular weight between crosslinks
- m d,a :
-
Mass of dry hydrogel in air
- m i :
-
Initial relaxed hydrogel mass
- MIC:
-
Minimum inhibitory concertation
- M n :
-
Number average molecular weight
- M r :
-
Molecular weight of the repeating unit
- m r,b :
-
Mass of relaxed hydrogel in butanol
- MRSA:
-
methicillin-resistant Staphylococcus aureus
- m s,b :
-
Mass of swollen hydrogel in butanol
- m t :
-
Swollen mass at a given time
- p :
-
Statistical p value
- PBS:
-
Phosphate buffered saline
- PEG:
-
Poly(ethylene glycol)
- PEGDA:
-
Poly(ethylene glycol) diacrylate
- P i :
-
Permeability coefficient
- q :
-
Hydrogel mass swelling ratio
- SSTI:
-
Skin and soft tissue infection
- t lag :
-
Lag time to steady state conditions
- UV:
-
Ultraviolet
- v :
-
Specific volume of the polymer
- V1 :
-
Molar volume of the swelling agent, water
- Δm:
-
Change in mass (%)
- ν2,r :
-
Polymer volume fraction in the relaxed state
- ν2,s :
-
Polymer volume fraction in the swollen state
- ξ:
-
Mesh size of the hydrogel
- ρb :
-
Density of 1-butanol
- ρp :
-
Density of polymer
- χ:
-
Flory polymer-solvent interaction factor
References
Tun K, Shurko JF, Ryan L, Lee GC. Age-based health and economic burden of skin and soft tissue infections in the United States, 2000 and 2012. PLoS One. 2018;13(11):e0206893–3.
Kaye KS, Petty LA, Shorr AF, Zilberberg MD. Current epidemiology, etiology, and burden of acute skin infections in the United States. Clin Infect Dis. 2019;68(Suppl 3):S193–9.
Ioannou P, Tsagkaraki E, Athanasaki A, Tsioutis C, Gikas A. Gram-negative Bacteria as emerging pathogens affecting mortality in skin and soft tissue infections. Hippokratia. 2018;22(1):23–8.
Bassetti M, Vena A, Croxatto A, Righi E, Guery B. How to manage pseudomonas aeruginosa infections. Drugs Context. 2018;7:212527–7.
Leong HN, Kurup A, Tan MY, Kwa ALH, Liau KH, Wilcox MH. Management of Complicated Skin and Soft Tissue Infections with a special focus on the role of newer antibiotics. Infect Drug Resist. 2018;11:1959–74.
Stein GE, Wells EM. The importance of tissue penetration in achieving successful antimicrobial treatment of nosocomial pneumonia and complicated skin and soft-tissue infections caused by methicillin-resistant staphylococcus aureus: vancomycin and linezolid. Curr Med Res Opin. 2010;26(3):571–88.
Fish DN. Meropenem in the treatment of complicated skin and soft tissue infections. Ther Clin Risk Manag. 2006;2(4):401–15.
Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229–41.
Rand BC, Penn-Barwell JG, Wenke JC. Combined local and systemic antibiotic delivery improves eradication of wound contamination: an animal experimental model of contaminated fracture. Bone Joint J. 2015;97-B(10):1423–7.
Siddiqui AR, Bernstein JM. Chronic wound infection: facts and controversies. Clin Dermatol. 2010;28(5):519–26.
Ki V, Rotstein C. Bacterial Skin and Soft Tissue Infections in Adults: A Review of Their Epidemiology, Pathogenesis, Diagnosis, Treatment and Site of Care. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale. 2008;19(2):173–184.
Yao K, Bae L, Yew WP. Post-operative wound management. Aust Fam Physician. 2013;42(12):867–70.
Murphy PS, Evans GRD. Advances in wound healing: a review of current wound healing products. Plast Surg Int. 2012;2012:190436–6.
Lin CC, Anseth KS. Peg hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26(3):631–43.
Li J, Mooney DJ. Designing Hydrogels for Controlled Drug Delivery. Nature Reviews Materials. 2016;1(12):16071.
Gupta B, Agarwal R, Alam MS. Hydrogels for wound healing applications. In: Rimmer S, editor. Biomedical Hydrogels: Woodhead Publishing; 2011. p. 184–227.
Chen SL, Fu RH, Liao SF, Liu SP, Lin SZ, Wang YC. A peg-based hydrogel for effective wound care management. Cell Transplant. 2018;27(2):275–84.
Andreopoulos FM, Persaud I. Delivery of basic fibroblast growth factor (Bfgf) from Photoresponsive hydrogel scaffolds. Biomaterials. 2006;27(11):2468–76.
Liu SQ, Yang C, Huang Y, Ding X, Li Y, Fan WM, et al. Antimicrobial and antifouling hydrogels formed in situ from polycarbonate and poly(ethylene glycol) via Michael addition. Adv Mater. 2012;24(48):6484–9.
Buwalda SJ, Vermonden T, Hennink WE. Hydrogels for therapeutic delivery: current developments and future directions. Biomacromolecules. 2017;18(2):316–30.
Lipsky BA, Napolitano LM, Moran GJ, Vo L, Nicholson S, Kim M. Inappropriate initial antibiotic treatment for complicated skin and soft tissue infections in hospitalized patients: incidence and associated factors. Diagn Microbiol Infect Dis. 2014;79(2):273–9.
Yang K, Han Q, Chen B, Zheng Y, Zhang K, Li Q, et al. Antimicrobial hydrogels: promising materials for medical application. Int J Nanomedicine. 2018;13:2217–63.
Buhrman JS, Rayahin JE, Köllmer M, Gemeinhart RA. In-house preparation of hydrogels for batch affinity purification of glutathione S-transferase tagged recombinant proteins. BMC Biotechnol. 2012;12:63.
Rayahin JE, Buhrman JS, Gemeinhart RA. Melittin-glutathione S-transferase fusion protein exhibits anti-inflammatory properties and minimal toxicity. Eur J Pharm Sci. 2014;65:112–21.
Paka GD, Ramassamy C. Optimization of curcumin-loaded peg-Plga nanoparticles by Gsh functionalization: investigation of the internalization pathway in neuronal cells. Mol Pharm. 2017;14(1):93–106.
Sen S, Bonfio C, Mansy SS, Cowan JA. Investigation of glutathione-derived electrostatic and hydrogen-bonding interactions and their role in defining Grx5 [2fe-2s] cluster optical spectra and transfer chemistry. J Biol Inorg Chem. 2018;23(2):241–52.
Buhrman JS, Cook LC, Rayahin JE, Federle MJ, Gemeinhart RA. Proteolytically activated anti-bacterial hydrogel microspheres. J Control Release. 2013;171(3):288–95.
Lutgring JD, Machado MJ, Benahmed FH, Conville P, Shawar RM, Patel J, Brown AC. Fda-Cdc Antimicrobial Resistance Isolate Bank: A Publicly Available Resource to Support Research, Development, and Regulatory Requirements. J Clin Microbiol. 2018;56(2).
Buhrman JS. Recombinant Protein Immobilization and Controlled-Release Mediated by a Non-Covalent Protein Anchor. Ph.D. Dissertation in Biopharmaceutical Sciences from the University of Illinois at Chicago (2015).
Ross AE, Tang MY, Gemeinhart RA. Effects of molecular weight and loading on matrix Metalloproteinase-2 mediated release from poly(ethylene glycol) Diacrylate hydrogels. AAPS J. 2012;14(3):482–90.
Gustafson CT, Boakye-Agyeman F, Brinkman CL, Reid JM, Patel R, Bajzer Z, et al. Controlled delivery of vancomycin via charged hydrogels. PLoS One. 2016;11(1):e0146401–1.
Mendez A, Chagastelles P, Palma E, Nardi N, Schapoval E. Thermal and alkaline stability of Meropenem: degradation products and cytotoxicity. Int J Pharm. 2008;350(1–2):95–102.
Bergman P, Linde C, Putsep K, Pohanka A, Normark S, Henriques-Normark B, et al. Studies on the antibacterial effects of statins--in vitro and in vivo. PLoS One. 2011;6(8):e24394.
Grip O, Janciauskiene S, Lindgren S. Pravastatin Down-regulates inflammatory mediators in human monocytes in vitro. Eur J Pharmacol. 2000;410(1):83–92.
Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing [S]. Clsi Supplement M100. In.: Clinical and Laboratory Standards Institute Wayne, PA; 2018.
O'Donnell K, Boyd A, Meenan BJ. Controlling Fluid Diffusion and Release through Mixed-Molecular-Weight Poly(Ethylene) Glycol Diacrylate (Pegda) Hydrogels. Materials (Basel). 2019;12(20).
Baietto L, Corcione S, Pacini G, Perri GD, D'Avolio A, De Rosa FG. A 30-years review on pharmacokinetics of antibiotics: is the right time for pharmacogenetics? Curr Drug Metab. 2014;15(6):581–98.
Xu H, Paxton JW, Wu Z. Enhanced Ph-responsiveness, cellular trafficking, cytotoxicity and long-circulation of Pegylated liposomes with post-insertion technique using gemcitabine as a model drug. Pharm Res. 2015;32(7):2428–38.
Duan JZ, Riviere K, Marroum P. In vivo bioequivalence and in vitro similarity factor (F2) for dissolution profile comparisons of extended release formulations: how and when do they match? Pharm Res. 2011;28(5):1144–56.
Sumon ZE, Berenson CS, Sellick JA, Bulman ZP, Tsuji BT, Mergenhagen KA. Successful cure of Daptomycin-non-susceptible, vancomycin-intermediate staphylococcus aureus prosthetic aortic valve endocarditis directed by synergistic in vitro time-kill study. Infect Dis (Lond). 2019;51(4):287–92.
Wenzler E, Santarossa M, Meyer KA, Harrington AT, Reid GE, Clark NM, Albarillo FS, Bulman ZP. In Vitro Pharmacodynamic Analyses Help Guide the Treatment of Multidrug-Resistant Enterococcus faecium and Carbapenem-Resistant Enterobacter cloacae Bacteremia in a Liver Transplant Patient. Open Forum Infect Dis. 2020;7(1):ofz545.
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–92.
Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Asp Med. 2009;30(1–2):1–12.
Zarembinski TI, Doty NJ, Erickson IE, Srinivas R, Wirostko BM, Tew WP. Thiolated Hyaluronan-based hydrogels crosslinked using oxidized glutathione: an injectable matrix designed for ophthalmic applications. Acta Biomater. 2014;10(1):94–103.
Li J, Shu Y, Hao T, Wang Y, Qian Y, Duan C, et al. A chitosan-glutathione based injectable hydrogel for suppression of oxidative stress damage in cardiomyocytes. Biomaterials. 2013;34(36):9071–81.
Bertoni S, Albertini B, Facchini C, Prata C, Passerini N. Glutathione-Loaded Solid Lipid Microparticles as Innovative Delivery System for Oral Antioxidant Therapy. Pharmaceutics. 2019;11(8).
Amsden B. Solute diffusion within hydrogels. Mechanisms and Models Macromolecules. 1998;31(23):8382–95.
Peppas NA, Reinhart CT. Solute diffusion in swollen membranes. Part I. a new theory. J Membr Sci. 1983;15(3):275–87.
Raghuwanshi VS, Garnier G. Characterisation of hydrogels: linking the Nano to the microscale. Adv Colloid Interf Sci. 2019;274:102044.
Kozlowska M, Goclon J, Rodziewicz P. Intramolecular hydrogen bonds in low-molecular-weight polyethylene glycol. Chemphyschem. 2016;17(8):1143–53.
Krezel A, Bal W. Structure-function relationships in glutathione and its analogues. Org Biomol Chem. 2003;1(22):3885–90.
Lyon RP, Atkins WM. Self-assembly and gelation of oxidized glutathione in organic solvents. J Am Chem Soc. 2001;123(19):4408–13.
Plaut BS, Davies DJ, Meakin BJ, Richardson NE. The mechanism of interaction between chlorhexidine Digluconate and poly(2-hydroxyethyl methacrylate). J Pharm Pharmacol. 1981;33(2):82–8.
Gehrke SH, Fisher JP, Palasis M, Lund ME. Factors determining hydrogel permeability. Ann N Y Acad Sci. 1997;831:179–207.
Ye F, Baldursdottir S, Hvidt S, Jensen H, Larsen SW, Yaghmur A, et al. Role of electrostatic interactions on the transport of Druglike molecules in hydrogel-based articular cartilage mimics: implications for drug delivery. Mol Pharm. 2016;13(3):819–28.
Koetting MC, Peters JT, Steichen SD, Peppas NA. Stimulus-responsive hydrogels: theory, modern advances, and applications. Mater Sci Eng R Rep. 2015;93:1–49.
Gao M, Gawel K, Stokke BT. Polyelectrolyte and Antipolyelectrolyte effects in swelling of Polyampholyte and Polyzwitterionic charge balanced and charge offset hydrogels. Eur Polym J. 2014;53:65–74.
Acknowledgements and Disclosures
The authors would like to acknowledge and thank Alec C. Thompson for his help in preparing hydrogels, Shitalben R. Patel for her help conducting the microbiology experiments, and Dr. Zackery P. Bulman for generous sharing of instrumentation and feedback. The authors also thank Catherine F. Dial and Timothy D. Langridge for their support and feedback during the development of this work. Karol Sokolowski: Methodology; Conceptualization; Investigation; Formal Analysis; Writing-Original Draft. Hai M. Pham: Investigation; Formal Analysis; Writing-Review & Editing. Eric Wenzler: Methodology; Formal Analysis; Supervision; Writing-Review & Editing. Richard Gemeinhart: Methodology; Conceptualization; Formal Analysis; Supervision; Writing-Review & Editing; Funding Acquisition. This work was supported in part by the W.C. and May Preble Deiss Fund for Biomedical Research (to K.S.). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR15482 from the National Center for Research Resources, NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request. E.W. serves on the speaker’s bureau for Melinta Therapeutics, Astellas Pharma, and Allergan Plc., and on the advisory board for GenMark Diagnostics and Shionogi. All other authors certify no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
Supplemental chemical schema and results include hydrogel swell behavior in isotonic PBS, agar-well diffusion data against carbapenem-susceptible P. aeruginosa, representative images of hydrogels, and physical characteristics of slab-cut hydrogels. Supplementary data to this article can be found online at TBD. (PDF 1128 kb)
Rights and permissions
About this article
Cite this article
Sokolowski, K., Pham, H.M., Wenzler, E. et al. Glutathione-Conjugated Hydrogels: Flexible Vehicles for Personalized Treatment of Bacterial Infections. Pharm Res 38, 1247–1261 (2021). https://doi.org/10.1007/s11095-021-03057-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03057-1