Abstract
Purpose
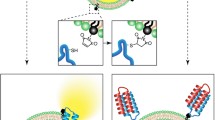
To develop a novel, target agnostic liposome click membrane permeability assay (LCMPA) using liposome encapsulating copper free click reagent dibenzo cyclooctyne biotin (DBCO-Biotin) to conjugate azido modified peptides that may effectively translocate from extravesicular space into the liposome lumen.
Method
DBCO-Biotin liposomes were prepared with egg phosphatidylcholine and cholesterol by lipid film rehydration, freeze/thaw followed by extrusion. Size of DBCO-Biotin liposomes were characterized with dynamic light scattering.
Results
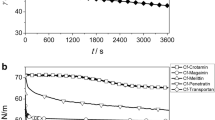
The permeable peptides representing energy independent mechanism of permeability showed higher biotinylation in LCMPA. Individual peptide permeability results from LCMPA correlated well with shifts in potency in cellular versus biochemical assays (i.e., cellular/ biochemical ratio) demonstrating quantitative correlation to intracellular barrier in intact cells.
Conclusion
The study provides a novel membrane permeability assay that has potential to evaluate energy independent transport of diverse peptides.




Similar content being viewed by others
Change history
05 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11095-021-03033-9
References
Nielsen DS, Shepherd NE, Xu W, Lucke AJ, Stoermer MJ, Fairlie DP. Orally absorbed cyclic peptides. Chem Rev. 2017;117(12):8094–128.
Vinogradov AA, Yin YZ, Suga H. Macrocyclic peptides as drug candidates: recent Progress and remaining challenges. J Am Chem Soc. 2019;141(10):4167–81.
Ahlbach CL, Lexa KW, Bockus AT, Chen V, Crews P, Jacobson MP, et al. Beyond cyclosporine a: conformation-dependent passive membrane permeabilities of cyclic peptide natural products. Future Med Chem. 2015;7(16):2121–30.
Walport LJ, Obexer R, Suga H. Strategies for transitioning macrocyclic peptides to cell permeable drug leads. Curr Opin Biotech. 2017;48:242–50.
Josephson K, Ricardo A, Szostak JW. mRNA display: from basic principles to macrocycle drug discovery. Drug Discov Today. 2014;19(4):388–99.
Matsson P, Doak BC, Over B, Kihlberg J. Cell permeability beyond the rule of 5. Adv Drug Deliver Rev. 2016;101:42–61.
Mateus A, Gordon LJ, Wayne GJ, Almqvist H, Axelsson H, Seashore-Ludlow B, et al. Prediction of intracellular exposure bridges the gap between target- and cell-based drug discovery. Proc Natl Acad Sci U S A. 2017;114(30):E6231–E9.
Sawyer TK, Partridge AW, Kaan HYK, Juang YC, Lim S, Johannes C, et al. Macrocyclic alpha helical peptide therapeutic modality: a perspective of learnings and challenges. Bioorg Med Chem. 2018;26(10):2807–15.
Treyer A, Mateus A, Wisniewski JR, Boriss H, Matsson P, Artursson P. Intracellular drug bioavailability: effect of neutral lipids and phospholipids. Mol Pharm. 2018;15(6):2224–33.
Treyer A, Ullah M, Parrott N, Molitor B, Fowler S, Artursson P. Impact of intracellular concentrations on metabolic drug-drug interaction studies. AAPS J. 2019;21(5):77.
Boehm M, Beaumont K, Jones R, Kalgutkar AS, Zhang L, Atkinson K, et al. Discovery of potent and orally bioavailable macrocyclic peptide-Peptoid hybrid CXCR7 modulators. J Med Chem. 2017;60(23):9653–63.
Kwon YU, Kodadek T. Quantitative evaluation of the relative cell permeability of peptoids and peptides. J Am Chem Soc. 2007;129(6):1508–9.
Schwochert J, Turner R, Thang M, Berkeley RF, Ponkey AR, Rodriguez KM, et al. Peptide to Peptoid substitutions increase cell permeability in cyclic Hexapeptides. Org Lett. 2015;17(12):2928–31.
Berben P, Bauer-Brandl A, Brandl M, Faller B, Flaten GE, Jacobsen AC, et al. Drug permeability profiling using cell-free permeation tools: overview and applications. Eur J Pharm Sci. 2018;119:219–33.
Li AP. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov Today. 2001;6(7):357–66.
Peraro L, Kritzer JA. Emerging methods and design principles for cell-penetrant peptides. Angew Chem Int Ed Engl. 2018;57(37):11868–81.
Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J Med Chem. 1998;41(7):1007–10.
Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. Second-generation difluorinated cyclooctynes for copper-free click chemistry. J Am Chem Soc. 2008;130(34):11486–93.
Erazo-Oliveras A, Najjar K, Truong D, Wang TY, Brock DJ, Prater AR, et al. The late endosome and its lipid BMP act as gateways for efficient cytosolic access of the delivery agent dfTAT and its macromolecular cargos. Cell Chem Biol. 2016;23(5):598–607.
Allolio C, Magarkar A, Jurkiewicz P, Baxova K, Javanainen M, Mason PE, et al. Arginine-rich cell-penetrating peptides induce membrane multilamellarity and subsequently enter via formation of a fusion pore. Proc Natl Acad Sci U S A. 2018;115(47):11923–8.
Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J Biol Chem. 2005;280(15):15300–6.
Brock DJ, Kondow-McConaghy HM, Hager EC, Pellois JP. Endosomal escape and cytosolic penetration of macromolecules mediated by synthetic delivery agents. Bioconjug Chem. 2019;30(2):293–304.
Yang ST, Zaitseva E, Chernomordik LV, Melikov K. Cell-penetrating peptide induces leaky fusion of liposomes containing late endosome-specific anionic lipid. Biophys J. 2010;99(8):2525–33.
Avdeef A. Physicochemical profiling (solubility, permeability and charge state). Curr Top Med Chem. 2001;1(4):277–351.
Chang YS, Graves B, Guerlavais V, Tovar C, Packman K, To KH, et al. Stapled alpha-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc Natl Acad Sci U S A. 2013;110(36):E3445–54.
Chu Q, Moellering RE, Hilinski GJ, Kim YW, Grossmann TN, Yeh JTH, et al. Towards understanding cell penetration by stapled peptides. Medchemcomm. 2015;6(1):111–9.
Partridge AW, Kaan HYK, Juang YC, Sadruddin A, Lim S, Brown CJ, et al. Incorporation of putative helix-breaking amino acids in the design of novel stapled peptides: exploring biophysical and cellular permeability properties. Molecules. 2019;24(12).
Bird GH, Madani N, Perry AF, Princiotto AM, Supko JG, He X, et al. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc Natl Acad Sci U S A. 2010;107(32):14093–8.
Falavigna M, Klitgaard M, Brase C, Ternullo S, Skalko-Basnet N, Flaten GE. Mucus-PVPA (mucus phospholipid vesicle-based permeation assay): an artificial permeability tool for drug screening and formulation development. Int J Pharm. 2018;537(1–2):213–22.
Flaten GE, Dhanikula AB, Luthman K, Brandl M. Drug permeability across a phospholipid vesicle based barrier: a novel approach for studying passive diffusion. Eur J Pharm Sci. 2006;27(1):80–90.
Cruz J, Mihailescu M, Wiedman G, Herman K, Searson PC, Wimley WC, et al. A membrane-Translocating peptide penetrates into bilayers without significant bilayer perturbations. Biophys J. 2013;104(11):2419–28.
Fuselier T, Wimley WC. Spontaneous membrane Translocating peptides: the role of Leucine-arginine consensus motifs. Biophys J. 2017;113(4):835–46.
Marks JR, Placone J, Hristova K, Wimley WC. Spontaneous membrane-translocating peptides by orthogonal high-throughput screening. J Am Chem Soc. 2011;133(23):8995–9004.
White TR, Renzelman CM, Rand AC, Rezai T, McEwen CM, Gelev VM, et al. On-resin N-methylation of cyclic peptides for discovery of orally bioavailable scaffolds. Nat Chem Biol. 2011;7(11):810–7.
Gautreau A, Oguievetskaia K, Ungermann C. Function and regulation of the endosomal fusion and fission machineries. Cold Spring Harb Perspect Biol. 2014;6(3).
Ostrowicz CW, Meiringer CT, Ungermann C. Yeast vacuole fusion: a model system for eukaryotic endomembrane dynamics. Autophagy. 2008;4(1):5–19.
Author information
Authors and Affiliations
Contributions
None
Corresponding authors
Ethics declarations
Conflict of Interest
Authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to replace Fig. 1. with the correct figure.
Supplementary Information
ESM 1
(DOCX 558 kb)
Rights and permissions
About this article
Cite this article
Desai, T.J., Habulihaz, B., Cannon, J.R. et al. Liposome Click Membrane Permeability Assay for Identifying Permeable Peptides. Pharm Res 38, 843–850 (2021). https://doi.org/10.1007/s11095-021-03005-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03005-z