Abstract
Purpose
Penetration enhancers are necessary to overcome a formidable barrier function of the stratum corneum in the development of topical formulations. Recently, non-lamella liquid crystal (NLLC)-forming lipids such as glycerol monooleate and phytantriol (PHY) are gaining increasing attention as a novel skin permeation enhancer. In the present study, fluorescein sodium (FL-Na) was used as a model hydrophilic drug, and acryl-base pressure-sensitive adhesive (PSA) tape containing NLLC forming lipids, mono-O-(5,9,13-trimethyl-4-tetradecenyl) glycerol ester (MGE) or PHY, was prepared to enhance drug permeation through the skin.
Methods
A PSA patch containing FL-Na was prepared by mixing FL-Na entrapped in NLLC and acrylic polymer. FL permeation through excised hairless rat skin, and also human skin, was investigated. Changes in lipid structure, folding/unfolding state of keratin in the stratum corneum, and penetration of MGE into the stratum corneum were investigated using confocal Raman microscopy.
Results
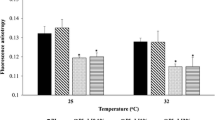
Enhanced FL permeation was observed by the application of a PSA patch containing MGE and PHY. Especially, dramatically enhancement effect was confirmed by 15% of MGE contained formulation. Penetration of MGE provided diminished orthorhombic crystal structure and a peak shift of the aliphatic CH3 vibration of keratin chains toward lower wavenumbers.
Conclusion
The present results suggested that the formulation development by adding MGE may be useful for improving the skin permeation of mal-permeable drugs such as hydrophilic drugs.









Similar content being viewed by others
Abbreviations
- ER:
-
Enhancement ratio
- FL:
-
Fluorescein sodium
- MGE:
-
Mono-O-(5,9,13-trimethyl-4-tetradecenyl) glycerol ester
- MW:
-
Molecular weight
- NLLC:
-
Non-lamella liquid crystal
- PET:
-
Polyethylene terephthalate
- PHY:
-
Phytantriol
- PSA:
-
Pressure-sensitive adhesive
- Q8h :
-
Cumulative amount of FL permeated over 8 h
- SAXS:
-
Small angle X-ray scattering
- TDDS:
-
Transdermal drug delivery system
References
Pastore MN, Kalia YN, Horstmann M, Roberts MS. Transdermal patches: history, development and pharmacology. Br J Pharmacol. 2015;172(9):2179–209.
Kováčik A, Kopečná M, Vávrová K. Permeation enhancers in transdermal drug delivery: benefits and limitations. Expert Opin Drug Deliv. 2020;17(2):145–55.
Lane ME. Skin penetration enhancers. Int J Pharm. 2013;447(1–2):12–21.
Zhai J, Fong C, Tran N, Drummond CJ. Non-lamellar lyotropic liquid crystalline lipid nanoparticles for the next generation of nanomedicine. ACS Nano. 2019;13(6):6178–206.
Barriga HMG, Holme MN, Stevens MM. Cubosomes: the next generation of smart lipid nanoparticles? Angew Chemie - Int Ed. 2019;58(10):2958–78.
Kim DH, Jahn A, Cho SJ, Kim JS, Ki MH, Kim DD. Lyotropic liquid crystal systems in drug delivery: a review. J Pharm Investig. 2015;45(1):1–11.
Yamada K, Yamashita J, Todo H, Miyamoto K, Hashimoto S, Tokudome Y, et al. Preparation and evaluation of liquid-crystal formulations with skin-permeation-enhancing abilities for entrapped drugs. J Oleo Sci. 2010;60(1):31–40.
Kadhum WR, Sekiguchi S, Hijikuro I, Todo H, Sugibayashi K. A novel chemical enhancer approach for transdermal drug delivery with C17-monoglycerol ester liquid crystal-forming lipid. J Oleo Sci. 2017;66(5):443–54.
Kadhum WR, Hada T, Hijikuro I, Todo H, Sugibayashi K. Development and optimization of orally and topically applied liquid crystal drug formulations. J Oleo Sci. 2017;66(9):939–50.
Shan QQ, Jiang XJ, Wang FY, Shu ZX, Gui SY. Cubic and hexagonal liquid crystals as drug carriers for the transdermal delivery of triptolide. Drug Deliv. 2019;26(1):490–8.
Wan J, Wang, S Mei, Gui, Z Ping, Yang, Z Zhuan, Shan, Q Qian, Chu, X Qin, et al. Phytantriol-based lyotropic liquid crystal as a transdermal delivery system. Eur J Pharm Sci 2018;125(1):93–101.
Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technolo Today. 2000;3(9):318–26.
Hato M, Minamikawa H, Salkar RA, Matsutani S. Alkylglycosides with an isoprenoid-type hydrophobic chain can afford greater control of aqueous phase structures at low temperatures. Langmuir. 2002;18(9):3425–9.
Hato M, Yamashita I, Kato T, Abe Y. Aqueous phase behavior of a 1-O-Phytanyl-β-D-xyloside/water system. Glycolipid-based bicontinuous cubic phases of crystallographic space groups Pn3m and Ia3d. Langmuir. 2004;20(26):11366–73.
Higuchi WI. Analysis of data on the medicament release from ointments. J Pharm Sci. 1962;51(8):802–4.
Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5(1):23–36.
Cilurzo F, Gennari CGM, Minghetti P. Adhesive properties: a critical issue in transdermal patch development. Expert Opin Drug Deliv. 2012;9(1):33–45.
Tojo K, Hikima T. Bioequivalence of marketed transdermal delivery systems for tulobuterol. Biol Pharm Bull. 2007;30(8):1576–9.
Okada A, Todo H, Hijikuro I, Itakura S, Sugibayashi K. Controlled release of a model hydrophilic high molecular weight compound from injectable non-lamellar liquid crystal formulations containing different types of phospholipids. Int J Pharm. 2020;577:118944. https://doi.org/10.1016/j.ijpharm.2019.118944.
Huang Y, Gui S. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Adv. 2018;8(13):6978–87.
Zabara A, Mezzenga R. Controlling molecular transport and sustained drug release in lipid-based liquid crystalline mesophases. J Control Release. 2014;188:31–43.
Gupta R, Dwadasi BS, Rai B, Mitragotri S. Effect of chemical permeation enhancers on skin permeability: in silico screening using molecular dynamics simulations. Sci Rep. 2019;9(1):1–11.
Choe CS, Schleusener J, Lademann J, Darvin ME. Human skin in vivo has a higher skin barrier function than porcine skin ex vivo—comprehensive Raman microscopic study of the stratum corneum. J Biophotonics. 2018;11(6):1–10.
Choe C, Schleusener J, Lademann J, Darvin ME. Keratin-water-NMF interaction as a three layer model in the human stratum corneum using in vivo confocal Raman microscopy. Sci Rep. 2017;7(1):1–13.
Barry BW. Mode of action of penetration enhancers in human skin. J Control Release. 1987;6(1):85–97. 26.
Yoshida S, Obata Y, Onuki Y, Utsumi S, Ohta N, Takahashi H, et al. Molecular interaction between intercellular lipids in the stratum corneum and l-menthol, as analyzed by synchrotron X-ray diffraction. Chem Pharm Bull. 2017;65(2):134–42.
Horita D, Hatta I, Yoshimoto M, Kitao Y, Todo H, Sugibayashi K. Molecular mechanisms of action of different concentrations of ethanol in water on ordered structures of intercellular lipids and soft keratin in the stratum corneum. Biochim Biophys Acta Biomembr. 2015;1848(5):1196–202.
Oshizaka T, Kikuchi K, Kadhum WR, Todo H, Hatanaka T, Wierzba K, et al. Estimation of skin concentrations of topically applied lidocaine at each depth profile. Int J Pharm. 2014;475(1–2):292–7.
Milewski M, Yerramreddy TR, Ghosh P, Crooks PA, Stinchcomb AL. In vitro permeation of a pegylated naltrexone prodrug across microneedle-treated skin. J Control Release. 2010;146(1):37–44.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suzuki, T., Aoki, T., Saito, M. et al. Enhancement of Skin Permeation of a Hydrophilic Drug from Acryl-Based Pressure-Sensitive Adhesive Tape. Pharm Res 38, 289–299 (2021). https://doi.org/10.1007/s11095-021-02996-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-02996-z