Abstract
Purpose
This prospective study aimed to evaluate the effects of genetic polymorphisms in sulindac-related metabolizing enzyme genes including FMO3 and AOX1 on the population pharmacokinetics of sulindac in 58 pregnant women with preterm labor.
Methods
Plasma samples were collected at 1.5, 4, and 10 h after first oral administration of sulindac. Plasma concentrations of sulindac and its active metabolite (sulindac sulfide) were determined, and pharmacokinetic analysis was performed with NONMEM 7.3.
Results
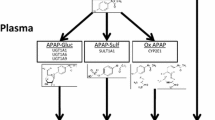
The mean maternal and gestational ages at the time of dosing were 32.5 ± 4.4 (range, 20–41) years and 27.4 ± 4.4 (range, 16.4–33.4) weeks, respectively. In the population pharmacokinetic analysis, one depot compartment model of sulindac with absorption lag time best described the data. The metabolism of sulindac and sulindac sulfide was described using Michaelis-Menten kinetics. In stepwise modeling, gestational age impacted volume of distribution (Vc), and FMO3 rs2266782 was shown by the Michaelis constant to affect conversion of sulindac sulfide to sulindac (KM32); these were retained in the final model.
Conclusions
Genetic polymorphisms of FMO3 and AOX1 could affect the pharmacokinetics of sulindac in women who undergo preterm labor. The results of this study could help clinicians develop individualized treatment plans for administering sulindac.




Similar content being viewed by others
Abbreviations
- AOX :
-
Aldehyde oxidase
- CWRES:
-
Conditional weighted residuals
- FMO3 :
-
Flavin-containing monooxygenase 3
- HWE :
-
Hardy-Weinberg equilibrium
- OFV:
-
Objective function value
- SNP :
-
Single nucleotide polymorphism
- SCM:
-
Stepwise covariate model
- VPC:
-
Visual predictive check
References
Simhan HN, Caritis SN. Prevention of preterm delivery. N Engl J Med. 2007;357:477–87.
Miyazaki C, Moreno GR, Ota E, Swa T, Oladapo OT, Mori R. Tocolysis for inhibiting preterm birth in extremely preterm birth, multiple gestations and in growth-restricted fetuses: a systematic review and meta-analysis. Reprod Health. 2016;13:4.
Davies NM, Watson MS. Clinical pharmacokinetics of sulindac. A dynamic old drug. Clin Pharmacokinet. 1997;32:437–59.
Eriksson LO, Bostrom H. Deactivation of sulindac-sulphide by human renal microsomes. Pharmacol Toxicol. 1988;62:177–83.
Duggan DE, Hooke KF, Hwang SS. Kinetics of the tissue distributions of sulindac and metabolites. Relevance to sites and rates of bioactivation. Drug Metab Dispos. 1980;8:241–6.
Miller MJ, Bednar MM, McGiff JC. Renal metabolism of sulindac: functional implications. J Pharmacol Exp Ther. 1984;231:449–56.
Ratnayake JH, Hanna PE, Anders MW, Duggan DE. Sulfoxide reduction. In vitro reduction of sulindac by rat hepatic cytosolic enzymes. Drug Metab Dispos. 1981;9:85–7.
Brunell D, Sagher D, Kesaraju S, Brot N, Weissbach H. Studies on the metabolism and biological activity of the Epimers of Sulindac. Drug Metab Dispos. 2011;39:1014–21.
Kuehl GE, Lampe JW, Potter JD, Bigler J. Glucuronidation of nonsteroidal anti-inflammatory drugs: identifying the enzymes responsible in human liver microsomes. Drug Metab Dispos. 2005;33:1027–35.
Duggan DE, Hare LE, Ditzler CA, Lei BW, Kwan KC. The disposition of sulindac. Clin Pharmacol Ther. 1977;21:326–35.
Dujovne C, Pitterman A, Vincek W, Dobrinska M. Enerohepatic circulation of sulindac and metabolites. Clin Pharmcol Ther. 1983;33:172–7.
Kitamura S, Ohashi KNK, Sugihara K, Hosokawa R, Akagawa Y, Ohta S. Extremely high drug-reductase activity based on aldehyde oxidase in monkey liver. Biol Pharm Bull. 2001;24:856–9.
Hisamuddin IM, Yang VW. Genetic polymorphisms of human flavin-containing monooxygenase 3: implications for drug metabolism and clinical perspectives. Pharmacogenomics. 2007;8:635–43.
Shimizu M, Yano H, Nagashima S, Murayama N, Zhang J, Cashman JR, et al. Effect of genetic variants of the human flavin-containing monooxygenase 3 on N- and S-oxygenation activities. Drug Metab Dispos. 2007;35:328–30.
Park S, Lee NR, Lee KE, Park JY, Kim YJ, Gwak HS. Effects of single-nucleotide polymorphisms of FMO3 and FMO6 genes on pharmacokinetic characteristics of sulindac sulfide in premature labor. Drug Metab Dispos. 2014;42:40–3.
Lee SC, Renwick AG. Sulphoxide reduction by rat and rabbit tissues in vitro. Biochem Pharmacol. 1995;49:1557–65.
Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9.
Park CS, Kang JH, Chung WG, Yi HG, Pie JE, Park DK, et al. Ethnic differences in allelic frequency of two flavin-containing monooxygenase 3 (FMO3) polymorphisms: linkage and effects on in vivo and in vitro FMO activities. Pharmacogenetics. 2002;12:77–80.
Koukouritaki SB, Poch MT, Henderson MC, Siddens LK, Krueger SK, VanDyke JE, et al. Identification and functional analysis of common human flavin-containing monooxygenase 3 genetic variants. J Pharmacol Exp Ther. 2007;320:266–73.
Lattard V, Zhang J, Tran Q, Furnes B, Schlenk D, Cashman JR. Two new polymorphisms of the FMO3 gene in Caucasian and African-American populations: comparative genetic and functional studies. Drug Metab Dispos. 2003;31:854–60.
Berg AK, Mandrekar SJ, Ziegler KL, Carlson EC, Szabo E, Ames MM, et al. Population pharmacokinetic model for cancer chemoprevention with sulindac in healthy subjects. J Clin Pharmacol. 2013;53:403–12.
Tang Y, Hu K, Huang W, Wang C, Liu Z, Chen Y, et al. Effects of FMO3 polymorphisms on pharmacokinetics of sulindac in Chinese healthy male volunteers. Biomed Res Int. 2017;4189678.
Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008.
Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18:152–61.
Davison JM, Dunlop W, Ezimokhai M. 24-hour creatinine clearance during the third trimester of normal pregnancy. Br J Obstet Gynaecol. 1980;87:106–9.
Tasnif Y, Morado J, Hebert MF. Pregnancy-related pharmacokinetic changes. Clin Pharmacol Ther. 2016;100:53–62.
Hytten FE, Paintin DB. Increase in plasma volume during normal pregnancy. J Obstet Gynaecol Br Emp. 1963;70:402–7.
Stormer E, Roots I, Brockmoller J. Benzydamine N-oxidation as an index reaction reflecting FMO activity in human liver microsomes and impact of FMO3 polymorphisms on enzyme activity. Br J Clin Pharmacol. 2000;50:553–61.
Wang L, Christopher LJ, Cui D, Li W, Iyer R, Humphreys WG, et al. Identification of the human enzymes involved in the oxidative metabolism of dasatinib: an effective approach for determining metabolite formation kinetics. Drug Metab Dispos. 2008;36:1828–39.
Lickteig AJ, Riley R, Melton RJ, Reitz BA, Fischer HD, Stevens JC. Expression and characterization of functional dog flavin-containing monooxygenase 3. Drug Metab Dispos. 2009;37:1987–90.
Manevski N, Balavenkatraman KK, Bertschi B, Swart P, Walles M, Camenisch G, et al. Aldehyde oxidase activity in fresh human skin. Drug Metab Dispos. 2014;42:2049–57.
Al-Salmy HS. Individual variation in hepatic aldehyde oxidase activity. IUBMB Life. 2001;51:249–53.
Hernandez D, Addou S, Lee D, Orengo C, Shephard EA, Phillips IR. Trimethylaminuria and a human FMO3 mutation database. Hum Mutat. 2003;22:209–13.
Hines RN, Cashman JR, Philpot RM, Williams DE, Ziegler DM. The mammalian flavin-containing monooxygenases: molecular characterization and regulation of expression. Toxicol Appl Pharmacol. 1994;125:1–6.
Mayatepek E, Flock B, Zschocke J. Benzydamine metabolism in vivo is impaired in patients with deficiency of flavin-containing monooxygenase 3. Pharmacogenetics. 2004;14:775–7.
Hisamuddin IM, Wehbi MA, Schmotzer B, Easley KA, Hylind LM, Giardiello FM, et al. Genetic polymorphisms of flavin monooxygenase 3 in sulindac-induced regression of colorectal adenomas in familial adenomatous polyposis. Cancer Epidemiol Biomark Prev. 2005;14:2366–9.
Hisamuddin IM, Wehbi MA, Chao A, Wyre HW, Hylind LM, Giardiello FM, et al. Genetic polymorphisms of human flavin monooxygenase 3 in sulindac-mediated primary chemoprevention of familial adenomatous polyposis. Clin Cancer Res. 2004;10:8357–62.
Kang JH, Chung WG, Lee KH, Park CS, Kang JS, Shin IC, et al. Phenotypes of flavin-containing monooxygenase activity determined by ranitidine N-oxidation are positively correlated with genotypes of linked FM03 gene mutations in a Korean population. Pharmacogenetics. 2000;10:67–78.
Catucci G, Bortolussi S, Rampolla G, Cusumano D, Gilardi G, Sadeghi S. Flavin-containing monooxygenase 3 polymorphic variants significantly affect clearance of tamoxifen and clomiphene.
Fields PA, Houseman DE. Decreases in activation energy and substrate affinity in cold-adapted A4-lactate dehydrogenase: evidence from the Antarctic notothenioid fish Chaenocephalus aceratus. Mol Biol Evol. 2004;21:2246–55.
Garattini E, Terao M. The role of aldehyde oxidase in drug metabolism. Expert Opin Drug Metab Toxicol. 2012;8:487–503.
Barr JT, Choughule K, Jones JP. Enzyme kinetics, inhibition, and regioselectivity of aldehyde oxidase. Methods Mol Biol. 2014;1113:167–86.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MEST) (grant number: NRF-2010-0022544) and the research fund of Hanyang University (grant number: HY-2018-N).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interests
No potential conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sung, J.W., Yun, Hy., Park, S. et al. Population Pharmacokinetics of Sulindac and Genetic Polymorphisms of FMO3 and AOX1 in Women with Preterm Labor. Pharm Res 37, 44 (2020). https://doi.org/10.1007/s11095-020-2765-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-2765-6