Abstract
Aim
The high doses of oral tacrolimus (TAC) (1,2) necessary to prevent acute rejection (AR) after vascularized composite allotransplantation (VCA) are associated with systemic adverse effects. The skin is the most antigenic tissue in VCA and the primary target of AR. However, the short-term use of topical TAC (Protopic®), as an off-label adjunct to oral TAC, to treat AR episodes pro re nata (PRN), has yielded inconsistent results. There is lack of data on the pharmacokinetics and tissue distribution of topical TAC in VCA, that hampers our understanding of the reasons for unreliable efficacy. Toward this goal, we evaluated the ability of topical TAC to achieve high local tissue concentrations at the site of application with low systemic concentrations.
Materials and Methods
We assessed the pharmacokinetics and tissue distribution of topical TAC (Protopic®, 0.03%) after single or repeated topical application in comparison to those after systemic delivery in rats. Animals received a single topical application of TAC ointment (Group 1) or an intravenous (IV) injection of TAC (Group 2) at a dose of 0.5 mg/kg. In another experiment, animals received daily topical application of TAC ointment (Group 3), or daily intraperitoneal (IP) injection of TAC (Group 4) at a dose of 0.5 mg/kg for 7 days. TAC concentrations in blood and tissues were analyzed by Liquid Chromatography–Mass Spectrometry (LC/MS-MS).
Results
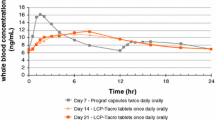
Following single topical administration, TAC was absorbed slowly with a Tmax of 4 h and an absolute bioavailability of 11%. The concentrations of TAC in skin and muscle were several folds higher than whole blood concentrations. Systemic levels remained subtherapeutic (< 3 ng/ml) with repeated once daily applications.
Conclusion
Topical application of TAC ointment (Protopic®, 0.03%) at a dose of 0.5 mg/kg/day provided high concentrations in the local tissues with low systemic exposure. Repeated topical administration of TAC is well tolerated with no local or systemic adverse effects. This study confirms the feasibility of topical application of TAC for site specific graft immunosuppression and enables future applications in VCA.








Similar content being viewed by others
References
Unadkat JV, Schnider JT, Feturi FG, Tsuji W, Bliley JM, Venkataramanan R, et al. Single Implantable FK506 Disk Prevents Rejection in Vascularized Composite Allotransplantation. Plast Reconstr Surg. 2017;139(2):403e–14e.
Feturi FG, Weinstock M, Zhao W, Zhang W, Schnider JT, Erbas VE, et al. Mycophenolic acid for topical immunosuppression in vascularized composite Allotransplantation: optimizing formulation and preliminary evaluation of bioavailability and pharmacokinetics. Front Surg. 2018;5:20.
Schneeberger S, Gorantla VS, Hautz T, Pulikkottil B, Margreiter R, Lee WP. Immunosuppression and rejection in human hand transplantation. Transplant Proc. 2009;41(2):472–5.
Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Gritsch HA, Corry RJ, et al. (1995) The superiority of tacrolimus in renal transplant recipients -- the Pittsburgh experience. Clin Transpl 199–205.
Gorantla VS, Barker JH, Jones JW Jr, Prabhune K, Maldonado C, Granger DK. Immunosuppressive agents in transplantation: mechanisms of action and current anti-rejection strategies. Microsurgery. 2000;20(8):420–9.
Offermann G. Five-year results of renal transplantation on immunosuppressive triple therapy with mycophenolate mofetil. Clin Transpl. 2003;17(1):43–6.
Schneeberger S, Gorantla VS, van Riet RP, Lanzetta M, Vereecken P, van Holder C, et al. Atypical acute rejection after hand transplantation. Am J Transplant. 2008;8(3):688–96.
Breidenbach WC, Gonzales NR, Kaufman CL, Klapheke M, Tobin GR, Gorantla VS. Outcomes of the first 2 American hand transplants at 8 and 6 years posttransplant. J Hand Surg Am. 2008;33(7):1039–47.
Ravindra KV, Buell JF, Kaufman CL, Blair B, Marvin M, Nagubandi R, et al. Hand transplantation in the United States: experience with 3 patients. Surgery. 2008;144(4):638–43 discussion 43-4.
Diaz-Siso JR, Fischer S, Sisk GC, Bueno E, Kueckelhaus M, Talbot S, et al. Initial experience of dual maintenance immunosuppression with steroid withdrawal in vascular composite tissue allotransplantation. Am J Transplant. 2015;15(5):1421–31.
Weissenbacher A, Cendales L, Morelon E, Petruzzo P, Brandacher G, Friend PJ, et al. Meeting report of the 13th congress of the International Society of Vascularized Composite Allotransplantation. Transplantation. 2018;102(8):1250–2.
Lanzetta M, Petruzzo P, Dubernard JM, Margreiter R, Schuind F, Breidenbach W, et al. Second report (1998-2006) of the international registry of hand and composite tissue transplantation. Transpl Immunol. 2007;18(1):1–6.
Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29(6):404–30.
Kelly PA, Burckart GJ, Venkataramanan R. Tacrolimus: a new immunosuppressive agent. Am J Health Syst Pharm. 1995;52(14):1521–35.
Capron A, Musuamba F, Latinne D, Mourad M, Lerut J, Haufroid V, et al. Validation of a liquid chromatography-mass spectrometric assay for tacrolimus in peripheral blood mononuclear cells. Ther Drug Monit. 2009;31(2):178–86.
Capron A, Lerut J, Verbaandert C, Mathys J, Ciccarelli O, Vanbinst R, et al. Validation of a liquid chromatography-mass spectrometric assay for tacrolimus in liver biopsies after hepatic transplantation: correlation with histopathologic staging of rejection. Ther Drug Monit. 2007;29(3):340–8.
Robbins NL, Wordsworth MJ, Parida BK, Kaplan B, Gorantla VS, Weitzel EK, et al. Is Skin the Most Allogenic Tissue in Vascularized Composite Allotransplantation and a Valid Monitor of the Deeper Tissues? Plast Reconstr Surg. 2019;143(4):880e–6e.
Petruzzo P, Dubernard JM. The international registry on hand and composite tissue allotransplantation. Clin Transpl. 2011:247–53.
Jose M. Caring for Australians with Renal I. The CARI guidelines. Calcineurin inhibitors in renal transplantation: adverse effects. Nephrology (Carlton). 2007;12(Suppl 1):S66–74.
Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80(2 Suppl):S254–64.
Bonatti H, Brandacher G, Margreiter R, Schneeberger S. Infectious complications in three double hand recipients: experience from a single center. Transplant Proc. 2009;41(2):517–20.
Schneeberger S, Lucchina S, Lanzetta M, Brandacher G, Bosmuller C, Steurer W, et al. Cytomegalovirus-related complications in human hand transplantation. Transplantation. 2005;80(4):441–7.
Solari MG, Washington KM, Sacks JM, Hautz T, Unadkat JV, Horibe EK, et al. Daily topical tacrolimus therapy prevents skin rejection in a rodent hind limb allograft model. Plast Reconstr Surg. 2009;123(2 Suppl):17S–25S.
Weissenbacher A, Hautz T, Pratschke J, Schneeberger S. Vascularized composite allografts and solid organ transplants: similarities and differences. Curr Opin Organ Transplant. 2013;18(6):640–4.
Schnider JT, Weinstock M, Plock JA, Solari MG, Venkataramanan R, Zheng XX, et al. Site-specific immunosuppression in vascularized composite allotransplantation: prospects and potential. Clin Dev Immunol. 2013;2013:495212.
Schneeberger S, Gorantla VS, Brandacher G, Zeevi A, Demetris AJ, Lunz JG, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg. 2013;257(2):345–51.
Diaz-Siso JR, Parker M, Bueno EM, Sisk GC, Pribaz JJ, Eriksson E, et al. Facial allotransplantation: a 3-year follow-up report. J Plast Reconstr Aesthet Surg. 2013;66(11):1458–63.
Kaufman CL, Bhutiani N, Ramirez A, Tien HY, Palazzo MD, Galvis E, et al. Current status of vascularized composite Allotransplantation. Am Surg. 2019;85(6):631–7.
Magalhaes OA, Marinho DR, Kwitko S. Topical 0.03% tacrolimus preventing rejection in high-risk corneal transplantation: a cohort study. Br J Ophthalmol. 2013;97(11):1395–8.
Reitamo S, Rustin M, Ruzicka T, Cambazard F, Kalimo K, Friedmann PS, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone butyrate ointment in adult patients with atopic dermatitis. J Allergy Clin Immunol. 2002;109(3):547–55.
Reitamo S, Harper J, Bos JD, Cambazard F, Bruijnzeel-Koomen C, Valk P, et al. 0.03% Tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double-blind controlled trial. Br J Dermatol. 2004;150(3):554–62.
Hanifin JM, Paller AS, Eichenfield L, Clark RA, Korman N, Weinstein G, et al. Efficacy and safety of tacrolimus ointment treatment for up to 4 years in patients with atopic dermatitis. J Am Acad Dermatol. 2005;53(2 Suppl 2):S186–94.
Chapman MS, Schachner LA, Breneman D, Boguniewicz M, Gold MH, Shull T, et al. Tacrolimus ointment 0.03% shows efficacy and safety in pediatric and adult patients with mild to moderate atopic dermatitis. J Am Acad Dermatol. 2005;53(2 Suppl 2):S177–85.
Paller A, Eichenfield LF, Leung DY, Stewart D, Appell M. A 12-week study of tacrolimus ointment for the treatment of atopic dermatitis in pediatric patients. J Am Acad Dermatol. 2001;44(1 Suppl):S47–57.
Soter NA, Fleischer AB Jr, Webster GF, Monroe E, Lawrence I. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(1 Suppl):S39–46.
Harper J, Smith C, Rubins A, Green A, Jackson K, Zigure S, et al. A multicenter study of the pharmacokinetics of tacrolimus ointment after first and repeated application to children with atopic dermatitis. J Invest Dermatol. 2005;124(4):695–9.
Krueger GG, Eichenfield L, Goodman JJ, Krafchik BR, Carlin CS, Pang ML, et al. Pharmacokinetics of tacrolimus following topical application of tacrolimus ointment in adult and pediatric patients with moderate to severe atopic dermatitis. J Drugs Dermatol. 2007;6(2):185–93.
Panhans-Gross A, Novak N, Kraft S, Bieber T. Human epidermal Langerhans' cells are targets for the immunosuppressive macrolide tacrolimus (FK506). J Allergy Clin Immunol. 2001;107(2):345–52.
Granstein RD, Smith L, Parrish JA. Prolongation of murine skin allograft survival by the systemic effects of 8-methoxypsoralen and long-wave ultraviolet radiation (PUVA). J Invest Dermatol. 1987;88(4):424–9.
Wollenberg A, Sharma S, von Bubnoff D, Geiger E, Haberstok J, Bieber T. Topical tacrolimus (FK506) leads to profound phenotypic and functional alterations of epidermal antigen-presenting dendritic cells in atopic dermatitis. J Allergy Clin Immunol. 2001;107(3):519–25.
Inceoglu S, Siemionow M, Chick L, Craven CM, Lister GD. The effect of combined immunosuppression with systemic low-dose cyclosporin and topical fluocinolone acetonide on the survival of rat hind-limb allografts. Ann Plast Surg. 1994;33(1):57–65.
Guo Y, Messner F, Etra JW, Beck SE, Kalsi R, Furtmuller GJ, et al. Efficacy of single-agent immunosuppressive regimens in a murine model of vascularized composite allotransplantation. Transpl Int. 2020;33(8):948–57.
Schweizer R, Taddeo A, Waldner M, Klein HJ, Fuchs N, Kamat P, et al. Adipose-derived stromal cell therapy combined with a short course nonmyeloablative conditioning promotes long-term graft tolerance in vascularized composite allotransplantation. Am J Transplant. 2020;20(5):1272–84.
Olariu R, Denoyelle J, Leclere FM, Dzhonova DV, Gajanayake T, Banz Y, et al. Intra-graft injection of tacrolimus promotes survival of vascularized composite allotransplantation. J Surg Res. 2017;218:49–57.
Gajanayake T, Olariu R, Leclere FM, Dhayani A, Yang Z, Bongoni AK, et al. A single localized dose of enzyme-responsive hydrogel improves long-term survival of a vascularized composite allograft. Sci Transl Med. 2014;6(249):249ra110.
Fujita T, Takahashi S, Yagihashi A, Jimbow K, Sato N. Prolonged survival of rat skin allograft by treatment with FK506 ointment. Transplantation. 1997;64(6):922–5.
Oishi N, Iwata H, Kobayashi N, Fujimoto K, Yamaura K. A survey on awareness of the "finger-tip unit" and medication guidance for the use of topical steroids among community pharmacists. Drug Discov Ther. 2019;13(3):128–32.
Dekio I, Morita E. The weight of a finger-tip unit of ointment in 5-gram tubes. J Dermatolog Treat. 2011;22(5):302–3.
Long CC, Finlay AY. The finger-tip unit--a new practical measure. Clin Exp Dermatol. 1991;16(6):444–7.
Kaneko T, Fujioka T, Suzuki Y, Nagano T, Sato Y, Asakura S, et al. Comparison of whole-blood tacrolimus concentrations measured by different immunoassay systems. J Clin Lab Anal. 2018;32(9):e22587.
Warty V, Zuckerman S, Venkataramanan R, Lever J, Fung J, Starzl T. FK506 measurement: comparison of different analytical methods. Ther Drug Monit. 1993;15(3):204–8.
Kirresh T, Tuteja S, Russo D, Brophy PD, Murry DJ. Development and validation of a liquid chromatography-mass spectrometric assay for simultaneous determination of tacrolimus and 13-O-desmethyl tacrolimus in rat kidney tissue. J Pharm Biomed Anal. 2017;136:32–7.
van der Merwe Y, Faust AE, Conner I, Gu X, Feturi F, Zhao W, et al. An elastomeric polymer matrix, PEUU-Tac, delivers bioactive Tacrolimus Transdurally to the CNS in rat. EBioMedicine. 2017;26:47–59.
Li L, Li X, Xu L, Sheng Y, Huang J, Zheng Q. Systematic evaluation of dose accumulation studies in clinical pharmacokinetics. Curr Drug Metab. 2013;14(5):605–15.
Lee WP, Yaremchuk MJ, Pan YC, Randolph MA, Tan CM, Weiland AJ. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg. 1991;87(3):401–11.
Gharb BB, Rampazzo A, Altuntas SH, Madajka M, Cwykiel J, Stratton J, et al. Effectiveness of topical immunosuppressants in prevention and treatment of rejection in face allotransplantation. Transplantation. 2013;95(10):1197–203.
Diehl R, Ferrara F, Muller C, Dreyer AY, McLeod DD, Fricke S, et al. Immunosuppression for in vivo research: state-of-the-art protocols and experimental approaches. Cell Mol Immunol. 2017;14(2):146–79.
Kezic S, Nielsen JB. Absorption of chemicals through compromised skin. Int Arch Occup Environ Health. 2009;82(6):677–88.
Lauerma AI, Surber C, Maibach HI. Absorption of topical tacrolimus (FK506) in vitro through human skin: comparison with cyclosporin a. Skin Pharmacol. 1997;10(5–6):230–4.
Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–9.
Nishioka Y, Yoshino H. Lymphatic targeting with nanoparticulate system. Adv Drug Deliv Rev. 2001;47(1):55–64.
Schneeberger S, Khalifian S, Brandacher G. Immunosuppression and monitoring of rejection in hand transplantation. Tech Hand Up Extrem Surg. 2013;17(4):208–14.
Khalifian S, Brazio PS, Mohan R, Shaffer C, Brandacher G, Barth RN, et al. Facial transplantation: the first 9 years. Lancet. 2014;384(9960):2153–63.
Akar Y, Yucel G, Durukan A, Yucel I, Arici G. Systemic toxicity of tacrolimus given by various routes and the response to dose reduction. Clin Exp Ophthalmol. 2005;33(1):53–9.
Mollison KW, Fey TA, Krause RA, Andrews JM, Bretheim PT, Cusick PK, et al. Nephrotoxicity studies of the immunosuppressants tacrolimus (FK506) and ascomycin in rat models. Toxicology. 1998;125(2–3):169–81.
Funding
This study was supported by extramural funding from the American Society of Surgery of the Hand (Award # 0043416) and intramural support from the Department of Pharmaceutical Sciences, Department of Plastic Surgery, University of Pittsburgh.
Author information
Authors and Affiliations
Contributions
Overall research idea (VG); conceptual approach, hypothesis (VG, RV, FF); experimental design (FF, VG, RV, MS); protocol development (FF, RV, VG); In vivo experiments and collection of data (FF, JS, PF, VE, SO, HS and LD); Tissue processing for drug level measurement (FF); Manuscript drafting (FF), Extensive manuscript editing (RV, VG), Additional input (MS, AS, JP, JU); Analysis of data and interpretation of results (RV, VG, FF, MS);. All authors read and approved the manuscript.
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vijay S. Gorantla and Raman Venkataramanan are the Co-Corresponding Authors
Rights and permissions
About this article
Cite this article
Feturi, F.G., Schnider, J.T., Fanzio, P.M. et al. Pharmacokinetics and Biodistribution of Tacrolimus after Topical Administration: Implications for Vascularized Composite Allotransplantation. Pharm Res 37, 222 (2020). https://doi.org/10.1007/s11095-020-02921-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02921-w