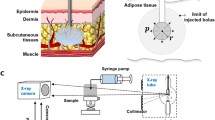
Abstract
Purpose
Injection devices for administration of biopharmaceuticals enable subcutaneous self-administration by patients. To meet patient specific capabilities, injection forces need to be characterized. We address the open question of whether tissue resistance significantly contributes to overall injection forces, especially for large injection volumes.
Methods
Subcutaneous tissue resistance was systematically quantified for injection volumes up to 11 mL depending on viscosity (1–20 mPa·s) and injection rates (0.025–0.2 mL/s) using Göttingen Minipigs as the animal model. The contribution of an artificially applied external force at the injection site simulating autoinjector needle cover depression was tested between 2.5–7.5 N.
Results
Tissue resistance reached average values of ~120 mbar for injection volumes up to 11 mL independent of viscosity and injection rate, and maximum values of 300 mbar were determined. Artificially applied external forces led to higher values, independent of the absolute applied force — maximum values of 1 bar were obtained when injecting 4.5 mL of the 20 mPa·s solution at an injection rate of 0.1 mL/s with the application of an artificial 5 N force, corresponding to ~450 mbar. All conditions yield defined injection sites suggesting tissue resistance is defined by mechanical properties of the subcutaneous tissue.
Conclusions
We set our results in relation to overall injection forces, concluding that maximum values in tissue resistance may cause challenges during subcutaneous injection when using injection devices.

Graphical abstract




Similar content being viewed by others
Change history
30 November 2020
Table 2 of the article ���Tissue Resistance during Large-Volume Injections in Subcutaneous Tissue of Minipigs��� encountered formatting errors in the (original) pdf version with misplacement of text and columns.
Abbreviations
- EFG:
-
External force generator
- i.m.:
-
Intramuscular
- s.c.:
-
Subcutaneous
- SD:
-
Standard deviation
- w/v :
-
Weight per volume
References
Narasimhan C, Mach H, Shameem M. High-dose monoclonal antibodies via the subcutaneous route: challenges and technical solutions, an industry perspective. Ther Deliv. 2012;3(7):889–900.
Mahler HC, Allmendinger A. Stability, formulation, and delivery of biopharmaceuticals. In: Vaughan T, Osbourn J, Jallal B, editors. Protein therapeutics, 1st ed. WILEY-VCH; 2017. p. 469–491.
Overcashier DE, Chan EK, Hsu CC. Technical considerations in the development of prefilled syringes for protein products. Am Pharm Rev. 2004;9:77–83.
Burckbuchler V, Mekhloufi G, Giteau AP, Grossiord JL, Huille S, Agnely F. Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions. Eur J Pharm Biopharm. 2010;76:351–6.
Allmendinger A, Fischer S, Huwyler J, Mahler HC, Schwarb E, Zarraga IE, et al. Rheological characterization and injection forces of concentrated protein formulations: an alternative predictive model for non-newtonian solutions. Eur J Pharm Biopharm. 2014;87(2):318–28.
Sheikhzadeh A, Yoon J, Formosa D, Domanska B, Morgan D, Schiff M. The effect of a new syringe design on the ability of rheumatoid arthritis patients to inject a biological medication. Appl Ergon. 2012;43(2):368–75.
Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93(6):1390–402.
Rathore N, Pranay P, Bernacki J, Eu B, Ji W, Walls E. Characterization of protein rheology and delivery forces for combination products. J Pharm Sci. 2012;101(12):4472–80.
Mathaes R, Koulov A, Joerg S, Mahler HC. Subcutaneous injection volume of biopharmaceuticals-pushing the boundaries. J Pharm Sci. 2016;105(8):2255–9.
European Medicines Agency. Firmagon: EPAR - Product Information. 2020 February 17. Available from: https://www.ema.europa.eu/en/documents/product-information/ firmagon-epar-product-information_en.pdf.
European Medicines Agency, Repatha: EPAR - Product Information. 2020 February 17. Available from: https://www.ema.europa.eu/en/documents/product-information/ repatha-epar-product-information_en.pdf.
Hamizi S, Freyer G, Bakrin N, Henin E, Mohtaram A, Le Saux O, et al. Subcutaneous trastuzumab: development of a new formulation for treatment of her2-positive early breast cancer. Onco Targets Ther. 2013;6:89–94.
Allmendinger A, Mueller R, Schwarb E, Chipperfield M, Huwyler J, Mahler HC, et al. Measuring tissue back-pressure--in vivo injection forces during subcutaneous injection. Pharm Res. 2015;32(7):2229–40.
Kinnunen HM, Mrsny RJ. Improving the outcomes of biopharmaceutical delivery via the subcutaneous route by understanding the chemical, physical and physiological properties of the subcutaneous injection site. J Control Release. 2014;182:22–32.
Locke KW, Maneval DC, LaBarre MJ. Enhanze® drug delivery technology: A novel approach to subcutaneous administration using recombinant human hyaluronidase PH20. Drug Deliv. 2019;26(1):98–106.
Comley K, Fleck NA. The toughness of adipose tissue: measurements and physical basis. J Biomech. 2010;43(9):1823–6.
Patte C, Pleus S, Wiegel C, Schiltges G, Jendrike N, Haug C, et al. Effect of infusion rate and indwelling time on tissue resistance pressure in small-volume subcutaneous infusion like in continuous subcutaneous insulin infusion. Diabetes Technol Ther. 2013;15(4):289–94.
Thomsen M, Hernandez-Garcia A, Mathiesen J, Poulsen M, Sorensen DN, Tarnow L, et al. Model study of the pressure build-up during subcutaneous injection. PLoS One. 2014;9(8):e104054.
Doughty DV, Clawson CZ, Lambert W, Subramony JA. Understanding subcutaneous tissue pressure for engineering injection devices for large-volume protein delivery. J Pharm Sci. 2016;105(7):2105–13.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The tables in the article appear correctly on Springerlink.com however Tables I and II in the online PDF do not download correctly. The spacing and data is not correct and do not match the author feedback file in JFLUX.
Electronic supplementary material
ESM 1
(DOCX 59.2 kb)
Rights and permissions
About this article
Cite this article
Allmendinger, A., Fischer, S. Tissue Resistance during Large-Volume Injections in Subcutaneous Tissue of Minipigs. Pharm Res 37, 184 (2020). https://doi.org/10.1007/s11095-020-02906-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02906-9