Abstract
Purpose
Hypoxia-inducible factor (HIF) is one of the critical components of the tumor microenvironment that is involved in tumor development. HIF-1α functionally and physically interacts with CDK1, 2, and 5 and stimulates the cell cycle progression and Cyclin-Dependent Kinase (CDK) expression. Therefore, hypoxic tumor microenvironment and CDK overexpression lead to increased cell cycle progression and tumor expansion. Therefore, we decided to suppress cancer cell expansion by blocking HIF-1α and CDK molecules.
Methods
In the present study, we used the carboxylated graphene oxide (CGO) conjugated with trimethyl chitosan (TMC) and hyaluronate (HA) nanoparticles (NPs) loaded with HIF-1α-siRNA and Dinaciclib, the CDK inhibitor, for silencing HIF-1α and blockade of CDKs in CD44-expressing cancer cells and evaluated the impact of combination therapy on proliferation, metastasis, apoptosis, and tumor growth.
Results
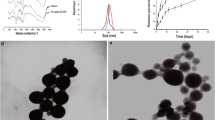
The results indicated that the manufactured NPs had conceivable physicochemical properties, high cellular uptake, and low toxicity. Moreover, combination therapy of cancer cells using CGO-TMC-HA NPs loaded with HIF-1α siRNA and Dinaciclib (SCH 727965) significantly suppressed the CDKs/HIF-1α and consequently, decreased the proliferation, migration, angiogenesis, and colony formation in tumor cells.
Conclusions
These results indicate the ability of CGO-TMC-HA NPs for dual drug/gene delivery in cancer treatment. Furthermore, the simultaneous inhibition of CDKs/HIF-1α can be considered as a novel anti-cancer treatment strategy; however, further research is needed to confirm this treatment in vivo.

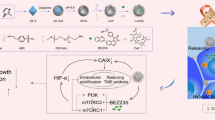
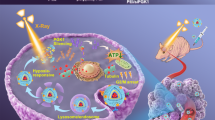
The suppression of HIF-1α and CDKs inhibits cancer growth. HIF-1α is overexpressed by the cells present in the tumor microenvironment. The hypoxic environment elevates mitochondrial ROS production and increases p38 MAP kinase, JAK/STAT, ERK, JNK, and Akt/PI3K signaling, resulting in cyclin accumulation and aberrant cell cycle progression. Furthermore, the overexpression of HIF-1α/CDK results in increased expression of genes such as BCL2, Bcl-xl, Ki-67, TGFβ, VEGF, FGF, MMP2, MMP9, and, HIF-1α and consequently raise the survival, proliferation, angiogenesis, metastasis, and invasion of tumor cells. In conclusion, HIF-1α-siRNA/Dinaciclib-loaded CGO-TMC-HA NPs can inhibit the tumor expansion by blockage of CDKs and HIF-1α (JAK: Janus kinase, STAT: Signal transducer and activator of transcription, MAPK: mitogen-activated protein kinase, ERK: extracellular signal-regulated kinase, JNK: c-Jun N-terminal kinase, PI3K: phosphatidylinositol 3-kinase).








Similar content being viewed by others
Change history
11 November 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11095-022-03432-6
References
Jadidi-Niaragh F, Atyabi F, Rastegari A, Kheshtchin N, Arab S, Hassannia H, et al. CD73 specific siRNA loaded chitosan lactate nanoparticles potentiate the antitumor effect of a dendritic cell vaccine in 4T1 breast cancer bearing mice. J Control Release. 2017;246:46–59.
Whiteside TJ. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–12.
Yuan Y, Jiang Y-C, Sun C-K, Chen Q-MJ. Role of the tumor microenvironment in tumor progression and the clinical applications. Oncol Rep. 2016;35(5):2499–515.
Quail DF, Joyce JAJ. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37.
Jadidi-Niaragh F, Atyabi F, Rastegari A, Mollarazi E, Kiani M, Razavi A, et al. Downregulation of CD73 in 4T1 breast cancer cells through siRNA-loaded chitosan-lactate nanoparticles. Tumor Biol. 2016;37(6):8403–12.
Wilson WR, Hay MPJ. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410.
Lequeux A, Noman MZ, Xiao M, Sauvage D, Van Moer K, Viry E, et al. Impact of hypoxic tumor microenvironment and tumor cell plasticity on the expression of immune checkpoints. Cancer Lett. 2019;458:13–20.
Kheshtchin N, Arab S, Ajami M, Mirzaei R, Ashourpour M, Mousavi N, et al. Inhibition of HIF-1α enhances anti-tumor effects of dendritic cell-based vaccination in a mouse model of breast cancer. Cancer Immunol Immunother. 2016;65(10):1159–67.
Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18(1):157.
Masoud GN, Li WJ. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–89.
Adamaki M, Georgountzou A, Moschovi MJ. Cancer and the cellular response to hypoxia. Pediatr Therapeut S. 2012;1:002.
Chouaib S, Noman M, Kosmatopoulos K, Curran MJ. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene. 2017;36(4):439–45.
Izadi S, Nikkhoo A, Hojjat-Farsangi M, Namdar A, Azizi G, Mohammadi H, et al. CDK1 in breast Cancer: implications for Theranostic potential. Anti Cancer Agents Med Chem. 2020;20:758–67.
Sharma R, Kumar D, Jha NK, Jha SK, Ambasta RK, Kumar PJ. Re-expression of cell cycle markers in aged neurons and muscles: whether cells should divide or die? Biochimica et Biophysica Acta -Molecular Basis of Disease. 2017;1863(1):324–36.
Mu W, Liu L-ZJ. Reactive oxygen species signaling in cancer development. Reactive Oxygen Species. 2017;4(10):251 –265-251–265.
Li Q, Mao M, Qiu Y, Liu G, Sheng T, Yu X, Wang S, Zhu DJ. Key role of ROS in the process of 15-lipoxygenase/15-hydroxyeicosatetraenoiccid-induced pulmonary vascular remodeling in hypoxia pulmonary hypertension. PLoS One. 2016;11(2).
Galanis A, Pappa A, Giannakakis A, Lanitis E, Dangaj D, Sandaltzopoulos RJ. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett. 2008;266(1):12–20.
Warfel NA, Dolloff NG, Dicker DT, Malysz J, El-Deiry WSJ. CDK1 stabilizes HIF-1α via direct phosphorylation of Ser668 to promote tumor growth. Cell Cycle. 2013;12(23):3689–701.
Hubbi ME, Gilkes DM, Hu H, Ahmed I, Semenza GLJ. Cyclin-dependent kinases regulate lysosomal degradation of hypoxia-inducible factor 1α to promote cell-cycle progression. Proc Natl Acad Sci. 2014;111(32):E3325–34.
Herzog J. Cyclin-dependent kinase 5 stabilizes the hypoxia-inducible factor in hepatocellular carcinoma. In.: lmu; 2015.
Herzog J, Ehrlich SM, Pfitzer L, Liebl J, Fröhlich T, Arnold GJ, et al. Cyclin-dependent kinase 5 stabilizes hypoxia-inducible factor-1α: a novel approach for inhibiting angiogenesis in hepatocellular carcinoma. Oncotarget. 2016;7(19):27108–21.
Lenjisa JL, Tadesse S, Khair NZ, Kumarasiri M, Yu M, Albrecht H, et al. CDK5 in oncology: recent advances and future prospects. Future Med Chem. 2017;9(16):1939–62.
Swadi RR, Sampat K, Herrmann A, Losty PD, See V, Moss DJJ. CDK inhibitors reduce cell proliferation and reverse hypoxia-induced metastasis of neuroblastoma tumours in a chick embryo model. Sci Rep. 2019;9(1):1–15.
Tai WJ. Current aspects of siRNA bioconjugate for in vitro and in vivo delivery. Molecules. 2019;24(12):2211.
Serrano-Sevilla I, Artiga Á, Mitchell SG, De Matteis L, de la Fuente JMJ. Natural polysaccharides for siRNA delivery: Nanocarriers based on chitosan, hyaluronic acid, and their derivatives. Molecules. 2019;24(14):2570.
Hosseini M, Haji-Fatahaliha M, Jadidi-Niaragh F, Majidi J, Yousefi M. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artificial cells, nanomedicine, and biotechnology. 2016;44(4):1051–61.
Singh ZJ. Applications and toxicity of graphene family nanomaterials and their composites. Nanotechnology, science applications. 2016;9:15.
Zhang B, Wei P, Zhou Z, Wei TJ. Interactions of graphene with mammalian cells: molecular mechanisms and biomedical insights. Adv Drug Deliv Rev. 2016;105:145–62.
Seabra AB, Paula AJ, de Lima R, Alves OL, Durán NJ. Nanotoxicity of graphene and graphene oxide. Chem Res Toxicol. 2014;27(2):159–68.
Ma X, Lee S, Fei X, Fang G, Huynh T, Chong Y, et al. Inhibition of the proteasome activity by graphene oxide contributes to its cytotoxicity. Nanotoxicology. 2018;12(2):185–200.
Liu J, Dong J, Zhang T, Peng QJ. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J Control Release. 2018;286:64–73.
Jin C, Wang F, Tang Y, Zhang X, Wang J, Yang YJ. Distribution of graphene oxide and TiO 2-graphene oxide composite in A549 cells. Biol Trace Elem Res. 2014;159(1–3):393–8.
Liao K-H, Lin Y-S, Macosko CW, Haynes CLJ. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS applied materials interfaces. 2011;3(7):2607–15.
Kurapati R, Russier J, Squillaci MA, Treossi E, Ménard-Moyon C, Del Rio-Castillo AE, et al. Dispersibility-dependent biodegradation of graphene oxide by myeloperoxidase. Small. 2015;11(32):3985–94.
Cardia MC, Carta AR, Caboni P, Maccioni AM, Erbì S, Boi L, et al. Trimethyl chitosan hydrogel nanoparticles for progesterone delivery in neurodegenerative disorders. Pharmaceutics. 2019;11(12):657.
Nikkhoo A, Rostami N, Farhadi S, Esmaily M, Ardebili SM, Atyabi F, et al. Codelivery of STAT3 siRNA and BV6 by carboxymethyl dextran trimethyl chitosan nanoparticles suppresses cancer cell progression. Int J Pharm. 2020;119236.
Hashemi V, Ahmadi A, Malakotikhah F, Chaleshtari MG, Moornani MB, Masjedi A, et al. Silencing of p68 and STAT3 synergistically diminishes cancer progression. Life Sci. 2020;117499.
Masjedi A, Ahmadi A, Atyabi F, Farhadi S, Irandoust M, Khazaei-Poul Y, et al. Silencing of IL-6 and STAT3 by siRNA loaded hyaluronate-N, N, N-trimethyl chitosan nanoparticles potently reduces cancer cell progression. Int J Biol Macromol. 2020;149:487–500.
Xia T, Cheng H, Zhu YJ, Science L. Knockdown of hypoxia-inducible factor-1 alpha reduces proliferation, induces apoptosis and attenuates the aggressive phenotype of retinoblastoma WERI-Rb-1 cells under hypoxic conditions. Annals of Clinical. 2014;44(2):134–44.
Assali A, Akhavan O, Adeli M, Razzazan S, Dinarvand R, Zanganeh S, et al. Multifunctional core-shell nanoplatforms (gold@ graphene oxide) with mediated NIR thermal therapy to promote miRNA delivery. Nanomedicine: Nanotechnology, Biology Medicine. 2018;14(6):1891–903.
Polnok A, Borchard G, Verhoef J, Sarisuta N, Junginger HJ. Influence of methylation process on the degree of quaternization of N-trimethyl chitosan chloride. European Journal of Pharmaceutics Biopharmaceutics. 2004;57(1):77–83.
Tekie FSM, Kiani M, Zakerian A, Pilevarian F, Assali A, Soleimani M, et al. Nano polyelectrolyte complexes of carboxymethyl dextran and chitosan to improve chitosan-mediated delivery of miR-145. Carbohydr Polym. 2017;159:66–75.
Hassannia H, Ghasemi Chaleshtari M, Atyabi F, Nosouhian M, Masjedi A, Hojjat-Farsangi M, et al. Blockage of immune checkpoint molecules increases T-cell priming potential of dendritic cell vaccine. Immunology. 2020;159(1):75–87.
Mu X, Lu H, Fan L, Yan S, Hu KJ. Efficient delivery of therapeutic siRNA with nanoparticles induces apoptosis in prostate cancer cells. J Nanomater. 2018;2018:1–10.
Shi J, Wang L, Zhang J, Ma R, Gao J, Liu Y, et al. A tumor-targeting near-infrared laser-triggered drug delivery system based on GO@ Ag nanoparticles for chemo-photothermal therapy and X-ray imaging. Biomaterials. 2014;35(22):5847–61.
Ghalamfarsa G, Rastegari A, Atyabi F, Hassannia H, Hojjat-Farsangi M, Ghanbari A, et al. Anti-angiogenic effects of CD73-specific siRNA-loaded nanoparticles in breast cancer-bearing mice. J Cell Physiol. 2018;233(10):7165–77.
Katas H, Amin M, Iqbal MC, Moideen N, Ng LY, Baharudin M, et al. Cell growth inhibition effect of DsiRNA vectorised by pectin-coated chitosan-graphene oxide nanocomposites as potential therapy for colon cancer. J Nanomater. 2017;2017:1–12.
Zhu H, Gao L, Jiang X, Liu R, Wei Y, Wang Y, et al. Positively charged graphene oxide nanoparticle: precisely label the plasma membrane of live cell and sensitively monitor extracellular pH in situ. Chem Commun. 2014;50(28):3695–8.
Chen J, Wang X, Chen TJ. Facile and green reduction of covalently PEGylated nanographene oxide via a ‘water-only’route for high-efficiency photothermal therapy. Nanoscale Res Lett. 2014;9(1):1–10.
Jiang W, Chen J, Gong C, Wang Y, Gao Y, Yuan YJ. Intravenous delivery of enzalutamide based on high drug loading multifunctional graphene oxide nanoparticles for castration-resistant prostate cancer therapy. Journal of Nanobiotechnology. 2020;18(1):1–12.
Li J, Ge X, Cui C, Zhang Y, Wang Y, Wang X, et al. Preparation and characterization of functionalized graphene oxide carrier for siRNA delivery. Int J Mol Sci. 2018;19(10):3202.
Yang C, Chan KK, Xu G, Yin M, Lin G, Wang X, et al. Biodegradable polymer-coated multifunctional graphene quantum dots for light-triggered synergetic therapy of pancreatic cancer. ACS applied materials interfaces. 2018;11(3):2768–81.
Jane EP, Premkumar D, Cavaleri J, Rajasekar T, Sutera P, Pollack I. Dinaciclib, a CDK inhibitor promotes proteasomal degradation of mcl-1 and enhances ABT-737 mediated cell death in malignant human glioma cell lines. In: AACR. 2016.
Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010;9(8):2344–53.
Johnson AJ, Yeh Y-Y, Smith LL, Wagner AJ, Hessler J, Gupta S, et al. The novel cyclin-dependent kinase inhibitor dinaciclib (SCH727965) promotes apoptosis and abrogates microenvironmental cytokine protection in chronic lymphocytic leukemia cells. Leukemia. 2012;26(12):2554–7.
Höring E, Montraveta A, Heine S, Kleih M, Schaaf L, Vöhringer MC, et al. Dual targeting of MCL1 and NOXA as effective strategy for treatment of mantle cell lymphoma. Br J Haematol. 2017;177(4):557–61.
Chen N, Chen X, Huang R, Zeng H, Gong J, Meng W, et al. BCL-xL is a target gene regulated by hypoxia-inducible factor-1α. J Biol Chem. 2009;284(15):10004–12.
Bose P, Simmons GL, Grant SJ. Cyclin-dependent kinase inhibitor therapy for hematologic malignancies. Expert Opin Investig Drugs. 2013;22(6):723–38.
Rello-Varona S, Fuentes-Guirado M, López-Alemany R, Contreras-Pérez A, Mulet-Margalef N, García-Monclús S, et al. Bcl-x L inhibition enhances Dinaciclib-induced cell death in soft-tissue sarcomas. Sci Rep. 2019;9(1):1–15.
Booher RN, Hatch H, Dolinski BM, Nguyen T, Harmonay L, Al-Assaad A-S, Ayers M, Nebozhyn M, Loboda A, Hirsch HAJ. MCL1 and BCL-xL levels in solid tumors are predictive of dinaciclib-induced apoptosis. PloS one. 2014;9(10).
Chen Y, Germano S, Clements C, Samuel J, Shelmani G, Jayne S, et al. Pro-survival signal inhibition by CDK inhibitor dinaciclib in chronic lymphocytic leukaemia. Br J Haematol. 2016;175(4):641–51.
Wong BS, Yoong SL, Jagusiak A, Panczyk T, Ho HK, Ang WH, et al. Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev. 2013;65(15):1964–2015.
Chen D, Dougherty CA, Zhu K, Hong HJ. Theranostic applications of carbon nanomaterials in cancer: focus on imaging and cargo delivery. J Control Release. 2015;210:230–45.
Tonelli FM, Goulart VA, Gomes KN, Ladeira MS, Santos AK, Lorençon E, et al. Graphene-based nanomaterials: biological and medical applications and toxicity. Nanomedicine: Nanotechnology, Biology. 2015;10(15):2423–50.
Mu Q, Su G, Li L, Gilbertson BO, Yu LH, Zhang Q, et al. Interfaces. Size-dependent cell uptake of protein-coated graphene oxide nanosheets. ACS applied materials. 2012;4(4):2259–66.
Rong P, Yang K, Srivastan A, Kiesewetter DO, Yue X, Wang F, et al. Photosensitizer loaded nano-graphene for multimodality imaging guided tumor photodynamic therapy. Theranostics. 2014;4(3):229–39.
Zheng X, Morgan J, Pandey SK, Chen Y, Tracy E, Baumann H, et al. Conjugation of 2-(1′-hexyloxyethyl)-2-devinylpyropheophorbide-a (HPPH) to carbohydrates changes its subcellular distribution and enhances photodynamic activity in vivo. J Med Chem. 2009;52(14):4306–18.
Lammel T, Boisseaux P, Fernández-Cruz M-L, Navas JMJ. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Particle fibre toxicology. 2013;10(1):27.
Feng L, Yang X, Shi X, Tan X, Peng R, Wang J, et al. Polyethylene glycol and polyethylenimine dual-functionalized nano-graphene oxide for photothermally enhanced gene delivery. Small. 2013;9(11):1989–97.
Yin D, Li Y, Lin H, Guo B, Du Y, Li X, et al. Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo. Nanotechnology, science. 2013;24(10):105102.
Zhang W, Xu W, Lan Y, He X, Liu K, Liang YJ. Antitumor effect of hyaluronic-acid-modified chitosan nanoparticles loaded with siRNA for targeted therapy for non-small cell lung cancer. Int J Nanomedicine. 2019;14:5287–301.
Hu H, Tang C, Yin CJ. Folate conjugated trimethyl chitosan/graphene oxide nanocomplexes as potential carriers for drug and gene delivery. Mater Lett. 2014;125:82–5.
Choi JY, Jang YS, Min SY, Song JYJ. Overexpression of MMP-9 and HIF-1α in breast cancer cells under hypoxic conditions. J Breast Cancer. 2011;14(2):88–95.
Liang H, Gu M, Yang C, Wang H, Wen X, Zhou QJ. Sevoflurane inhibits invasion and migration of lung cancer cells by inactivating the p38 MAPK signaling pathway. J Anesth. 2012;26(3):381–92.
Zhou T, Zhang B, Wei P, Du Y, Zhou H, Yu M, et al. Energy metabolism analysis reveals the mechanism of inhibition of breast cancer cell metastasis by PEG-modified graphene oxide nanosheets. Biomaterials. 2014;35(37):9833–43.
Zhou H, Zhang B, Zheng J, Yu M, Zhou T, Zhao K, et al. The inhibition of migration and invasion of cancer cells by graphene via the impairment of mitochondrial respiration. Biomaterials. 2014;35(5):1597–607.
Liu T, Li J, Wu X, Zhang S, Lu Z, Li G, et al. Transferrin-targeting redox hyperbranched poly (amido amine)-functionalized graphene oxide for sensitized chemotherapy combined with gene therapy to nasopharyngeal carcinoma. Drug delivery. 2019;26(1):744–55.
Bos R, van Diest PJ, van der Groep P, Shvarts A, Greijer AE, van der Wall EJ. Expression of hypoxia-inducible factor-1α and cell cycle proteins in invasive breast cancer are estrogen receptor related. Breast Cancer Res. 2004;6(4):R450.
Liebl J, Weitensteiner SB, Vereb G, Takács L, Fürst R, Vollmar AM, et al. Cyclin-dependent kinase 5 regulates endothelial cell migration and angiogenesis. J Biol Chem. 2010;285(46):35932–43.
Cao D, Hou M, Guan Y-s, Jiang M, Yang Y, Gou H-fJ. Expression of HIF-1alpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC cancer. 2009;9(1):432.
Mouriaux F, Sanschagrin F, Diorio C, Landreville S, Comoz F, Petit E, et al. Increased HIF-1α expression correlates with cell proliferation and vascular markers CD31 and VEGF-A in uveal melanoma. Investigative ophthalmology visual science. 2014;55(3):1277–83.
Nalwoga H, Ahmed L, Arnes JB, Wabinga H, Akslen LAJ. Strong expression of hypoxia-inducible factor-1α (HIF-1α) is associated with Axl expression and features of aggressive tumors in african breast cancer. PloS one. 2016;11(1).
Sun Q, Wang X, Cui C, Li J, Wang YJ. Doxorubicin and anti-VEGF siRNA co-delivery via nano-graphene oxide for enhanced cancer therapy in vitro and in vivo. Int J Nanomedicine. 2018;13:3713–28.
ACKNOWLEDGMENTS AND DISCLOSURES
We would like to thank the financial support of Tabriz University of Medical Sciences to perform thiswork (grant number: 61627), and Student Research Committee of Tabriz University of Medical Sciences (grant number: 61345). We are also thankful for the grant provided by the National Institute for Medical Research Development (NIMAD) for performing this study (Grant number: 976852).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
There is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11095-022-03432-6
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Izadi, S., Moslehi, A., Kheiry, H. et al. RETRACTED ARTICLE: Codelivery of HIF-1α siRNA and Dinaciclib by Carboxylated Graphene Oxide-Trimethyl Chitosan-Hyaluronate Nanoparticles Significantly Suppresses Cancer Cell Progression. Pharm Res 37, 196 (2020). https://doi.org/10.1007/s11095-020-02892-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02892-y