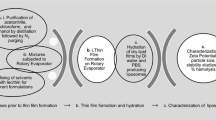
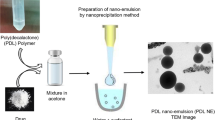
Abstract
Purpose
Complexities surrounding the manufacture and quality control of nanomedicines become increasingly apparent. This research article offers a case study to investigate how, at the laboratory scale, various stages of liposome and nanoparticle synthesis affect the amount of residual solvent found in the formulations. The objective is to bring insights on the reliability of each of these processes to provide final products which meet regulatory standards and facilitate identifying possible bottleneck early during the development process.
Methods
The residual solvent at various stages of preparation and purification was measured by headspace gas chromatography. Liposomes were prepared by two different methods with and without solvent. Polymer nanoparticles prepared via nanoprecipitation and purified by ultrafiltration were studied. The effects of purification by size exclusion chromatography and dialysis were also investigated.
Results
The complete removal of residual solvent requires processes which go beyond usual preparation methods.
Conclusions
This work might prove valuable as a reference for scientists of different fields to compare their own practices and streamline the translation of nanomedicines into efficacious and safe drug products.





Similar content being viewed by others
References
United States Pharmacopeial Convention. USP <467> Residual solvents. United States Pharmacopeia and National Formulary (USP 42-NF 37), United States Pharmacopeial Convention, Rockville, MD, 2019, pp. 1–25.
Witschiand C, Doelker E. Residual solvents in pharmaceutical products: acceptable limits, influences on physicochemical properties, analytical methods and documented values. Eur J Pharm Biopharm. 1997;43:215–42.
Lepeltier E, Bourgaux C, Amenitsch H, Rosilio V, Lepetre-Mouelhi S, Zouhiri F, et al. Influence of the nanoprecipitation conditions on the supramolecular structure of squalenoyled nanoparticles. Eur J Pharm Biopharm. 2015;96:89–95.
ICH Expert Working Group. ICH guideline Q3C (R6) on impurities: guideline for residual solvents Step 5, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) 2019, pp. 1–32.
William B, Noémie P, Brigitte E, Géraldine P. Supercritical fluid methods: an alternative to conventional methods to prepare liposomes. Chem Eng J. 2020;383:123106.
Kunastitchai S, Pichert L, Sarisuta N, Müller BW. Application of aerosol solvent extraction system (ASES) process for preparation of liposomes in a dry and reconstitutable form. Int J Pharm. 2006;316:93–101.
Koushikand K, Kompella UB. Preparation of large porous Deslorelin-PLGA microparticles with reduced residual solvent and cellular uptake using a supercritical carbon dioxide process. Pharm Res. 2004;21:524–35.
Hamdallah SI, Zoqlam R, Erfle P, Blyth M, Alkilany AM, Dietzel A, et al. Microfluidics for pharmaceutical nanoparticle fabrication: the truth and the myth. Int J Pharm. 2020;584:119408.
Anton N, Bally F, Serra CA, Ali A, Arntz Y, Mely Y, et al. A new microfluidic setup for precise control of the polymer nanoprecipitation process and lipophilic drug encapsulation. Soft Matter. 2012;8:10628–35.
Dimov N, Kastner E, Hussain M, Perrie Y, Szita N. Formation and purification of tailored liposomes for drug delivery using a module-based micro continuous-flow system. Sci Rep. 2017;7:12045.
Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3):e10143.
Ashton S, Song YH, Nolan J, Cadogan E, Murray J, Odedra R, et al. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci Transl Med. 2016;8(325):325ra17.
Bastiat G, Pritz CO, Roider C, Fouchet F, Lignières E, Jesacher A, et al. A new tool to ensure the fluorescent dye labeling stability of nanocarriers: a real challenge for fluorescence imaging. J Control Release. 2013;170:334–42.
Yamamoto S, Kasai T, Matsumoto M, Nishizawa T, Arito H, Nagano K, et al. Carcinogenicity and chronic toxicity in rats and mice exposed to chloroform by inhalation. J Occup Health. 2002;44:283–93.
Saillenfaitand AM, Sabaté JP. Comparative developmental toxicities of aliphatic nitriles: in vivo and in vitro observations. Toxicol Appl Pharmacol. 2000;163:149–63.
E.W.G. ICH. Impurities: Guidelines for residual solvents Q3C(R5), ICH, 2011, p. 25.
B'Hymer C. Residual solvent testing: a review of gas-chromatographic and alternative techniques. Pharm Res. 2003;20:337–44.
Bertrand N, Grenier P, Mahmoudi M, Lima EM, Appel EA, Dormont F, et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat Commun. 2017;8:777.
Brandl F, Bertrand N, Lima EM, Langer R. Nanoparticles with photoinduced precipitation for the extraction of pollutants from water and soil. Nat Commun. 2015;6:7765.
M.J. Hope, M.B. Bally, G. Webb, and P.R. Cullis. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochimica et Biophysica Acta. 812:55–65 (1985).
Bertrand N, Simard P, Leroux J-C. Serum-stable, long-circulating, pH-sensitive PEGylated liposomes. In: D'souza GG, editor. Liposomes: methods and protocols, vol. 1. Clifton, NJ: Humana Press; 2017. p. 193–207.
Colas J-C, Shi W, Rao VSNM, Omri A, Mozafari MR, Singh H. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron. 2007;38:841–7.
Pitorre M, Bastiat G, dit Chatel EM, Benoit J-P. Passive and specific targeting of lymph nodes: the influence of the administration route. Eur J Nanomed. 2015;7:121.
Bartlett GR. Phosphorous assay in column chromatography. J Biol Chem. 1959;234:466–8.
Chotard E, Mohammadi F, Julien P, Berthiaume L, Rudkowska I, Bertrand N. Drinkable lecithin vesicles to study the biological effects of individual hydrophobic macronutrients and food preferences. Food Chem. 2020;322:126736.
Fry DW, White JC, Goldman ID. Rapid separation of low molecular weight solutes from liposomes without dilution. Anal Biochem. 1978;90:809–15.
Bertrand N, Bouvet C, Moreau P, Leroux JC. Transmembrane pH-gradient liposomes to treat cardiovascular drug intoxication. ACS Nano. 2010;4:7552–8.
Scatchardand G, Raymond CL. Vapor—Liquid Equilibrium. II. Chloroform—Ethanol Mixtures at 35, 45 and 55°. J Am Chem Soc. 1938;60:1278–87.
Cui J, Li C, Deng Y, Wang Y, Wang W. Freeze-drying of liposomes using tertiary butyl alcohol/water cosolvent systems. Int J Pharm. 2006;312:131–6.
Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, et al. Formulation of functionalized PLGA–PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28:869–76.
Han E-J, Chung A-H, Oh I-J. Analysis of residual solvents in poly(lactide-co-glycolide) nanoparticles. J Pharm Investig. 2012;42:251–6.
Falkand RF, Randolph TW. Process variable implications for residual solvent removal and polymer morphology in the formation of gentamycin-loaded poly (L-lactide) microparticles. Pharm Res. 1998;15:1233–7.
Mayo AS, Ambati BK, Kompella UB. Gene delivery nanoparticles fabricated by supercritical fluid extraction of emulsions. Int J Pharm. 2010;387:278–85.
Herberger J, Murphy K, Munyakazi L, Cordia J, Westhaus E. Carbon dioxide extraction of residual solvents in poly(lactide-co-glycolide) microparticles. J Control Release. 2003;90:181–95.
Li Y, Zhao X, Zu Y, Zhang Y. Preparation and characterization of paclitaxel nanosuspension using novel emulsification method by combining high speed homogenizer and high pressure homogenization. Int J Pharm. 2015;490:324–33.
Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58:1688–713.
Author information
Authors and Affiliations
Contributions
NB designed the experiments. AD, FM, KG, CQ, PA and NB conducted the experiments and analyzed the results. NB prepared the figures and wrote the manuscript. GB, IR, NB contributed methods, funding and/or infrastructures. All authors read and commented the manuscript.
Corresponding author
Additional information
Amrita Dikpati, Farzad Mohammadi and Karine Greffard contributed equally to this work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 831 kb)
Rights and permissions
About this article
Cite this article
Dikpati, A., Mohammadi, F., Greffard, K. et al. Residual Solvents in Nanomedicine and Lipid-Based Drug Delivery Systems: a Case Study to Better Understand Processes. Pharm Res 37, 149 (2020). https://doi.org/10.1007/s11095-020-02877-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02877-x