Abstract
Purpose
Topical corticosteroids administration is commonly used for management of various ocular conditions especially those affecting the anterior segment of the eye. Poor solubility and limited pre-corneal residence time result in insufficient drug penetration to the outer (cornea and conjunctival-scleral) coats of the eye. This study aimed to prepare and evaluate cubosomes for prolonging residence time and enhancing ocular bioavailability of BDP.
Methods
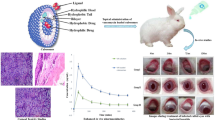
GMO-cubosomes were prepared using the top-down technique. Two stabilizers were investigated: poloxamer 407 and solulan C24. Particle size, EE %, polarized-light microscopy, TEM, in vitro release, transcorneal permeation, BCOP, histopathology and in vivo evaluation for treatment of uveitis in a rabbits’ model were studied.
Results
The prepared cubosomes were of nano-sizes (100 nm – 278 nm); EE% was around 94%. The cubosomes were confirmed by visualizing the “Maltese crosses” textures. Transcorneal permeation was significantly (p < 0.05) improved, compared to BDP-suspension (the control formulation). The optimized cubosomes F1P was incorporated in CMC gel (Cubo-gel). The prepared Cubo-gel formulations showed better rheological characteristics and high ocular tolerability. Superior anti-inflammatory properties were recorded for the Cubo-gel for treatment of endotoxin-induced uveitis in the rabbit model when compared to the control BDP-suspension.
Conclusions
Transcorneal permeation parameters Papp and flux and AUC0-10h markedly enhanced by up to 4-, 5.8-and 5.5-fold respectively, compared to the control BDP-suspension formulation. This study suggested that cubosomes/Cubo-gel could be an auspicious ocular delivery system for BDP that was able to effectively treat uveitis (a disease of the posterior segment of the eye).













Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- BCOP:
-
Bovine corneal opacity and permeability assay
- BDP:
-
Beclomethasone dipropionate
- CMC:
-
Carboxymethylcellulose
- EE%:
-
Entrapment efficiency percentage
- GMO:
-
Glyceryl monooleate
- Papp :
-
Apparent permeability coefficient
- TEM:
-
Transmission electron microscopy
References
Fel A, Aslangul E, Le Jeunne C. Eye and corticosteroid's use. Presse Med. 2012;41(4):414–21.
Abdelkader H, Alany R. Controlled and continuous release ocular drug delivery systems: pros and cons. Curr Drug Deliv. 2012;9(4):421–30.
Kwatra D, Mitra AK. Drug delivery in ocular diseases: Barriers and strategies. World J Pharmacol. 2013;2(4):78–83.
Järvinen K, Järvinen T, Urtti A. Ocular absorption following topical delivery. Adv Drug Deliv Rev. 1995;16(1):3–19.
Ozerdem U, Levi L, Cheng L, Song MK, Scher C, Freeman WR. Systemic toxicity of topical and periocular corticosteroid therapy in an 11-year-old male with posterior uveitis. Am J Ophthalmol. 2000;130(2):240–1.
McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55.
McCluskey PJ, Towle HM, Lightman S. Management of chronic uveitis. Bmj. 2000;320(7234):555–8.
Feiler DL, Srivastava SK, Pichi F, Bena J, Lowder CY. Resolution of noninfectious uveitic cystoid macular edema with topical difluprednate. Retina. 2017;37(5):844–50.
Abdelkader H, Alani AW, Alany RG. Recent advances in non-ionic surfactant vesicles (niosomes): self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug Deliv. 2014;21(2):87–100.
Mishra GP, Bagui M, Tamboli V, Mitra AK. Recent applications of liposomes in ophthalmic drug delivery. J Drug Deliv. 2011;2011.
Sharma A, Kumar L, Kumar P, Prasad N, Rastogi V. Niosomes: A Promising Approach in Drug Delivery Systems. J Drug Del Therap. 2019;9(4):635–42.
Karami Z, Hamidi M. Cubosomes: remarkable drug delivery potential. Drug Discov Today. 2016;21(5):789–801.
Khan S, Jain P, Jain S, Jain R, Bhargava S, Jain A. Topical delivery of erythromycin through cubosomes for acne. Pharm Nanotechnol. 2018;6(1):38–47.
Gaballa SA, El Garhy OH, Abdelkader H. Cubosomes: composition, preparation, and drug delivery applications. J Adv Biomed Pharm Sci. 2020;3(1):1–9.
Rizwan SB, Boyd BJ. Cubosomes: structure, preparation and use as an antigen delivery system. Subunit Vaccine Delivery: Springer; 2015. p. 125-40.
Boyd BJ, Whittaker DV, Khoo S-M, Davey G. Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int J Pharm. 2006;309(1-2):218–26.
Anbarasan B, Grace XF, Shanmuganathan S. An overview of cubosomes–smart drug delivery system. Sri Ramachandra J Med. 2015;8(1):1–4.
Gan L, Han S, Shen J, Zhu J, Zhu C, Zhang X, et al. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: improving preocular retention and ocular bioavailability. Int J Pharm. 2010;396(1-2):179–87.
Thadanki M, Kumari PS, Prabha KS. Overview of cubosomes: a nano particle. Int J Res Pharm Chem. 2011;1(3):535–41.
Sheskey PJ, Cook WG, Cable CG. Glyceryl monooleate. In: Sheskey PJ, Cook WG, Cable CG, editors. Handbook of Pharmaceutical Excipients 8th. Edition ed. London: Pharmaceutical Press; 2017. p. 411–3.
Kulkarni CV, Wachter W, Iglesias-Salto G, Engelskirchen S, Ahualli S. Monoolein: a magic lipid? Phys Chem Chem Phys. 2011;13(8):3004–21.
Zhang J, Wang S. Topical use of Coenzyme Q10-loaded liposomes coated with trimethyl chitosan: tolerance, precorneal retention and anti-cataract effect. Int J Pharm. 2009;372(1-2):66–75.
Gordon S, Young K, Wilson R, Rizwan S, Kemp R, Rades T, et al. Chitosan hydrogels containing liposomes and cubosomes as particulate sustained release vaccine delivery systems. J Liposome Res. 2012;22(3):193–204.
Brown HM, Storey G, George W. Beclomethasone dipropionate: a new steroid aerosol for the treatment of allergic asthma. Br Med J. 1972;1(5800):585–90.
Rizzello F, Mazza M, Salice M, Calabrese C, Calafiore A, Campieri M, et al. The safety of beclomethasone dipropionate in the treatment of ulcerative colitis. Expert Opin Drug Saf. 2018;17(9):963–9.
Weiss C. Materials characterisation of crystalline beclomethasone dipropionate: impact of manufacturing conditions on physicochemical properties 2018.
Shah TJ, Conway MD, Peyman GA. Intracameral dexamethasone injection in the treatment of cataract surgery induced inflammation: design, development, and place in therapy. Clin Ophthalmol. 2018;12:2223.
He Y, Yi W, Suino-Powell K, Zhou XE, Tolbert WD, Tang X, et al. Structures and mechanism for the design of highly potent glucocorticoids. Cell Res. 2014;24(6):713–26.
Sakagami M, Kinoshita W, Sakon K. Sato J-i, Makino Y. Mucoadhesive beclomethasone microspheres for powder inhalation: their pharmacokinetics and pharmacodynamics evaluation. J Control Release. 2002;80(1-3):207–18.
Esposito E, Cortesi R, Drechsler M, Paccamiccio L, Mariani P, Contado C, et al. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res. 2005;22(12):2163–73.
Verma P, Ahuja M. Cubic liquid crystalline nanoparticles: optimization and evaluation for ocular delivery of tropicamide. Drug Deliv. 2016;23(8):3043–54.
Hundekar YR, Saboji J, Patil S, Nanjwade B. Preparation and evaluation of diclofenac sodium cubosomes for percutaneous administration. WJPPS. 2014;3(1):523–39.
Lessnig W, Metz G, Spiegel W, Faust M, Junkers G. Apparatus for measuring surface tension. Google Patents; 1982.
Ali K, Bilal S. Surface tensions and thermodynamic parameters of surface formation of aqueous salt solutions: III. Aqueous solution of KCl, KBr and KI. Colloids Surf A Physicochem Eng Asp. 2009;337(1-3):194–9.
Abdelkader H. Design and Characterisation of Niosomes for Ocular Delivery of Naltrexone Hydrochloride 2011.
Berger N, Sachse A, Bender J, Schubert R, Brandl M. Filter extrusion of liposomes using different devices: comparison of liposome size, encapsulation efficiency, and process characteristics. Int J Pharm. 2001;223(1):55–68.
El-Enin HA, AL-Shanbari AH. Nanostructured liquid crystalline formulation as a remarkable new drug delivery system of anti-epileptic drugs for treating children patients. Saudi Pharm J. 2018;26(6):790–800.
Nasr M, Ghorab MK, Abdelazem A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm Sin B. 2015;5(1):79–88.
Peng X, Zhou Y, Han K, Qin L, Wu C. Preparation and in vitro study on diffusion of capsaicin cubosome. Zhongguo Zhong Yao Za Zhi. 2014;39(4):644–7.
Prasanna B, Shetty A, Nadh T, Gopinath B, Ahmed M. Development of new spectrophotometric methods for the simultaneous estimation of levosalbutamol sulfate and beclomethasone dipropionate in bulk drug and pharmaceutical formulations (rotacap). Int J PharmTech Res. 2012;4(2):791–8.
Han S, Shen J-Q, Gan Y, Geng H-M, Zhang X-X, Zhu C-L, et al. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol Sin. 2010;31(8):990.
Gaafar PM, Abdallah OY, Farid RM, Abdelkader H. Preparation, characterization and evaluation of novel elastic nano-sized niosomes (ethoniosomes) for ocular delivery of prednisolone. J Liposome Res. 2014;24(3):204–15.
Abdelkader H, Ismail S, Kamal A, Alany R. Preparation of niosomes as an ocular delivery system for naltrexone hydrochloride: physicochemical characterization. Die Pharm-Int J Pharm Sci. 2010;65(11):811–7.
Dong Y, Dong P, Huang D, Mei L, Xia Y, Wang Z, et al. Fabrication and characterization of silk fibroin-coated liposomes for ocular drug delivery. Eur J Pharm Biopharm. 2015;91:82–90.
Ali Z, Sharma PK, Warsi MH. Fabrication and Evaluation of Ketorolac Loaded Cubosome for Ocular Drug Delivery. J Appl Pharm Sci. 2016;6(09):204–8.
Yang Z, Peng X, Tan Y, Chen M, Zhu X, Feng M, et al. Optimization of the preparation process for an oral phytantriol-based amphotericin B cubosomes. J Nanomater. 2011;2011:4.
El-Kamel A. In vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleate. Int J Pharm. 2002;241(1):47–55.
Tatke A, Dudhipala N, Janga K, Balguri S, Avula B, Jablonski M, et al. In Situ Gel of Triamcinolone Acetonide-Loaded Solid Lipid Nanoparticles for Improved Topical Ocular Delivery: Tear Kinetics and Ocular Disposition Studies. J Nanomater. 2019;9(1):33.
Yang T, Cui F-D, Choi M-K, Cho J-W, Chung S-J, Shim C-K, et al. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm. 2007;338(1-2):317–26.
Abdelkader H, Ismail S, Kamal A, Alany RG. Design and evaluation of controlled-release niosomes and discomes for naltrexone hydrochloride ocular delivery. J Pharm Sci. 2011;100(5):1833–46.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15(1):25–35.
Peppas N. Analysis of Fickian and non-Fickian drug release from polymers. 1985.
Peppas NA, Sahlin JJ. A simple equation for the description of solute release .III. Coupling of diffusion and relaxation. Int J Pharm. 1989;57(2):169–72.
Abdelkader H, Longman MR, Alany RG, Pierscionek B. Phytosome-hyaluronic acid systems for ocular delivery of L-carnosine. Int J Nanomedicine. 2016;11:2815.
Li C, Chen R, Xu M, Qiao J, Yan L, Guo XD. Hyaluronic acid modified MPEG-b-PAE block copolymer aqueous micelles for efficient ophthalmic drug delivery of hydrophobic genistein. Drug Deliv. 2018;25(1):1258–65.
Abdelbary GA, Amin MM, Zakaria MY. Ocular ketoconazole-loaded proniosomal gels: formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017;24(1):309–19.
Dai Y, Zhou R, Liu L, Lu Y, Qi J, Wu W. Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeation. Int J Nanomedicine. 2013;8:1921.
Schoenwald RD, Huang HS. Corneal penetration behavior of β-blocking agents I: Physicochemical factors. J Pharm Sci. 1983;72(11):1266–72.
Ramsay E, del Amo EM, Toropainen E, Tengvall-Unadike U, Ranta V-P, Urtti A, et al. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur J Pharm Sci. 2018;119:83–9.
Attama A, Reichl S, Müller-Goymann C. Sustained release and permeation of timolol from surface-modified solid lipid nanoparticles through bioengineered human cornea. Curr Eye Res. 2009;34(8):698–705.
Abdelkader H, Ismail S, Hussein A, Wu Z, Al-Kassas R, Alany RG. Conjunctival and corneal tolerability assessment of ocular naltrexone niosomes and their ingredients on the hen's egg chorioallantoic membrane and excised bovine cornea models. Int J Pharm. 2012;432(1-2):1–10.
Pastor-Clerigues A, Serrano A, Milara J, Marti-Bonmati E, Lopez-Perez FJ, Garcia-Montanes S, et al. Evaluation of the ocular tolerance of three tacrolimus topical pharmaceutical preparations by bovine corneal opacity and permeability test. Curr Eye Res. 2016;41(7):890–6.
Cooper K, Earl L, Harbell J, Raabe H. Prediction of ocular irritancy of prototype shampoo formulations by the isolated rabbit eye (IRE) test and bovine corneal opacity and permeability (BCOP) assay. Toxicol in Vitro. 2001;15(2):95–103.
Kassem M, Rahman AA, Ghorab M, Ahmed M, Khalil R. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int J Pharm. 2007;340(1-2):126–33.
Alshamsan A, Kalam MA, Vakili MR, Binkhathlan Z, Raish M, Ali R, et al. Treatment of endotoxin-induced uveitis by topical application of cyclosporine a-loaded PolyGel™ in rabbit eyes. Int J Pharm. 2019;569:118573.
Baranano DE, Kim SJ, Edelhauser HF, Durairaj C, Kompella UB, Handa JT. Efficacy and pharmacokinetics of intravitreal non-steroidal anti-inflammatory drugs for intraocular inflammation. Br J Ophthalmol. 2009;93(10):1387–90.
Pignatello R, Bucolo C, Ferrara P, Maltese A, Puleo A, Puglisi G. Eudragit RS100® nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci. 2002;16(1-2):53–61.
Chen C-L, Chen J-T, Liang C-M, Tai M-C, Lu D-W, Chen Y-H. Silibinin treatment prevents endotoxin-induced uveitis in rats in vivo and in vitro. PLoS One. 2017;12(4):e0174971.
Bucci FA Jr, Waterbury LD, Amico LM. Prostaglandin E2 inhibition and aqueous concentration of ketorolac 0.4%(acular LS) and nepafenac 0.1%(nevanac) in patients undergoing phacoemulsification. Am J Ophthalmol. 2007;144(1):146–7.
Parks DJ, Cheung MK, Chan C-C, Roberge FG. The role of nitric oxide in uveitis. Arch Ophthalmol. 1994;112(4):544–6.
Griess P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt “Ueber einige Azoverbindungen”. Ber Deutsch Chem Ges. 1879;12(1):426–8.
Hajj-Ali RA, Lowder C, Mandell BF. Uveitis in the internist’s office: are a patient’s eye symptoms serious. Cleve Clin J Med. 2005;72(4):329–39.
El-Shazly LHM, El-Gohary AA, El-Hossary GG. Safety of intravitreal triamcinolone acetonide: an electrophysiologic and histopathological study in rabbits. Int J Ophthalmol. 2013;6(6):790–5.
Lancelot A, Sierra T, Serrano JL. Nanostructured liquid-crystalline particles for drug delivery. Expert Opin Drug Deliv. 2014;11(4):547–64.
Elnaggar YS, Etman SM, Abdelmonsif DA, Abdallah OY. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: pharmaceutical, biological, and toxicological studies. Int J Nanomedicine. 2015;10:5459.
Uchegbu I. Some aspects of the niosomal delivery of doxorubicin: University of London; 1994.
Alkholief M, Albasit H, Alhowyan A, Alshehri S, Raish M, Kalam MA, et al. Employing a PLGA-TPGS based nanoparticle to improve the ocular delivery of Acyclovir. Saudi Pharm J. 2018;27(2):293–302.
Pawar PK, Majumdar DK. Effect of formulation factors on in vitro permeation of moxifloxacin from aqueous drops through excised goat, sheep, and buffalo corneas. AAPS PharmSciTech. 2006;7(1):E89.
Rathore MS, Majumdar DK. Effect of formulation factors on in vitro transcorneal permeation of gatifloxacin from aqueous drops. AAPS PharmSciTech. 2006;7(3):E12.
Abdelkader H, Wu Z, Al-Kassas R, Alany RG. Niosomes and discomes for ocular delivery of naltrexone hydrochloride: morphological, rheological, spreading properties and photo-protective effects. Int J Pharm. 2012;433(1-2):142–8.
Singh Y. Martin’s physical pharmacy and pharmaceutical sciences. New Jersey: Department of Pharmaceutics Ernest Mario School of Pharmacy Rutgers, The State University of New Jersey. 2006.
Aldrich D, Bach CM, Brown W, Chambers W, Fleitman J, Hunt D, et al. Ophthalmic preparations. US Pharmacopeia. 2013;39(5):1–21.
Huang J, Peng T, Li Y, Zhan Z, Zeng Y, Huang Y, et al. Ocular cubosome drug delivery system for timolol maleate: preparation, characterization, cytotoxicity, ex vivo, and in vivo evaluation. AAPS PharmSciTech. 2017;18(8):2919–26.
Lai J, Chen J, Lu Y, Sun J, Hu F, Yin Z, et al. Glyceryl monooleate/poloxamer 407 cubic nanoparticles as oral drug delivery systems: I. In vitro evaluation and enhanced oral bioavailability of the poorly water-soluble drug simvastatin. AAPS PharmSciTech. 2009;10(3):960.
Spicer PT, Hayden KL, Lynch ML, Ofori-Boateng A, Burns JL. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir. 2001;17(19):5748–56.
Jain V, Swarnakar NK, Mishra PR, Verma A, Kaul A, Mishra AK, et al. Paclitaxel loaded PEGylated gleceryl monooleate based nanoparticulate carriers in chemotherapy. Biomaterials. 2012;33(29):7206–20.
Svensson O, Thuresson K, Arnebrant T. Interactions between drug delivery particles and mucin in solution and at interfaces. Langmuir. 2008;24(6):2573–9.
Manosroi A, Wongtrakul P, Manosroi J, Sakai H, Sugawara F, Yuasa M, et al. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Colloids Surf B: Biointerfaces. 2003;30(1-2):129–38.
Abdelkader H, Mansour HF. Comparative studies for ciprofloxacin hydrochloride pre-formed gels and thermally triggered (in situ) gels: in vitro and in vivo appraisal using a bacterial keratitis model in rabbits. Pharm Dev Technol. 2015;20(4):410–6.
Brogden R, Heel R, Speight T, Avery G. Beclomethasone dipropionate. Drugs. 1984;28(2):99–126.
Barnes PJ. Inhaled glucocorticoids for asthma. N Engl J Med. 1995;332(13):868–75.
Kwon TK, Hong SK, Kim J-C. In vitro skin permeation of cubosomes containing triclosan. J Ind Eng. 2012;18(1):563–7.
Chandaroy P, Sen A, Hui SW. Temperature-controlled content release from liposomes encapsulating Pluronic F127. J Control Release. 2001;76(1-2):27–37.
Charrueau C, Zandanel C. Drug delivery by polymer nanoparticles: the challenge of controlled release and evaluation. Polymer Nanoparticles for Nanomedicines: Springer; 2016. p. 439-503.
Guo C, Wang J, Cao F, Lee RJ, Zhai G. Lyotropic liquid crystal systems in drug delivery. Drug Discov Today. 2010;15(23-24):1032–40.
Boyd BJ, Whittaker DV, Khoo S-M, Davey G. Hexosomes formed from glycerate surfactants—Formulation as a colloidal carrier for irinotecan. Int J Pharm. 2006;318(1-2):154–62.
Peng X, Wen X, Pan X, Wang R, Chen B, Wu C. Design and in vitro evaluation of capsaicin transdermal controlled release cubic phase gels. AAPS PharmSciTech. 2010;11(3):1405–10.
Lopes LB, Speretta FF, Bentley MVL. Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. Eur J Pharm Sci. 2007;32(3):209–15.
Peng X, Zhou Y, Han K, Qin L, Dian L, Li G, et al. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des Devel Ther. 2015;9:4209.
Prinsen M, Koëter H. Justification of the enucleated eye test with eyes of slaughterhouse animals as an alternative to the Draize eye irritation test with rabbits. Food Chem Toxicol. 1993;31(1):69–76.
Curren RD, Harbell JW. In vitro alternatives for ocular irritation. Environ Health Perspect. 1998;106(suppl 2):485–92.
Abdelkader H, Pierscionek B, Carew M, Wu Z, Alany R. Critical appraisal of alternative irritation models: three decades of testing ophthalmic pharmaceuticals. Br Med Bull. 2015;113:59–71.
Verstraelen S, Jacobs A, De Wever B, Vanparys P. Improvement of the Bovine Corneal Opacity and Permeability (BCOP) assay as an in vitro alternative to the Draize rabbit eye irritation test. Toxicol in Vitro. 2013;27(4):1298–311.
Harbell J, Raabe H, Dobson T, Evans M, Curren R. Histopathology associated with opacity and permeability changes in bovine corneas in vitro. ATLA-Nottingham. 1999;27:347.
Harbell J, Mun G, Curren R, eds. Application of histological evaluation to enhance the bovine opacity and permeability (BCOP) assay. The 45th Annual Society of Toxicology Meeting, San Diego, CA, USA; 2006.
Sasaki H, Yamamura K, Nishida K, Nakamura J, Ichikawa M. Delivery of drugs to the eye by topical application. Prog Rettin Eye Res. 1996;15(2):583–620.
Maurice D, Mishima S. Ocular pharmacokinetics. Pharmacology of the Eye: Springer; 1984. p. 19-116.
Li J-C, Zhu N, Zhu J-X, Zhang W-J, Zhang H-M, Wang Q-Q, et al. Self-assembled cubic liquid crystalline nanoparticles for transdermal delivery of paeonol. Med Sci Monit. 2015;21:3298.
Kassem M, Attia M, Safwat S, El-Mahdy M. Preparation and evaluation of hydrocortisone multiple emulsions in rabbit's eye. Pharm Ind. 1994;56(6):584–8.
Li Q, Peng B, Whitcup SM, Jang SU, Chan C-C. Endotoxin induced uveitis in the mouse: susceptibility and genetic control. Exp Eye Res. 1995;61(5):629–32.
Shen J, Gan L, Zhu C, Zhang X, Dong Y, Jiang M, et al. Novel NSAIDs ophthalmic formulation: flurbiprofen axetil emulsion with low irritancy and improved anti-inflammation effect. Int J Pharm. 2011;412(1-2):115–22.
Lallemand F, Felt-Baeyens O, Besseghir K, Behar-Cohen F, Gurny R. Cyclosporine A delivery to the eye: a pharmaceutical challenge. Eur J Pharm Biopharm. 2003;56(3):307–18.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gaballa, S.A., El Garhy, O.H., Moharram, H. et al. Preparation and Evaluation of Cubosomes/Cubosomal Gels for Ocular Delivery of Beclomethasone Dipropionate for Management of Uveitis. Pharm Res 37, 198 (2020). https://doi.org/10.1007/s11095-020-02857-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02857-1