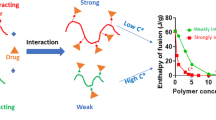
Abstract
Purpose
An investigation of underlying mechanisms of API-polymer interaction patterns has the potential to provide valuable insights for selecting appropriate formulations with superior physical stability and processability.
Materials and Methods
In this study, copovidone was used as a polymeric carrier for several model compounds including clotrimazole, nifedipine, and posaconazole. The varied chemical structures conferred the ability for the model compounds to form distinct interactions with copovidone. Rheology and nuclear magnetic resonance (NMR) were combined to investigate the molecular pattern and relative strength of active pharmaceutical ingredient (API)-polymer interactions. In addition, the impact of the interactions on formulation processability via hot melt extrusion (HME) and physical stability were evaluated.
Results
The rheological response of an API-polymer system was found to be highly sensitive to API-polymer interaction, depending both on API chemistry and API-polymer miscibility. In the systems studied, dispersed API induced a stronger plasticizer effect on the polymer matrix compared to crystalline/aggregated API. Correspondingly, the processing torque via HME showed a proportional relationship with the maximum complex viscosity of the API-polymer system. In order to quantitatively evaluate the relative strength of the API-polymer interaction, homogeneously dispersed API-polymer amorphous samples were prepared by HME at an elevated temperature. DSC, XRD, and rheology were employed to confirm the amorphous integrity and homogeneity of the resultant extrudates. Subsequently, the homogeneously dispersed API-polymer amorphous dispersions were interrogated by rheology and NMR to provide a qualitative and quantitative assessment of the nature of the API-polymer interaction, both macroscopically and microscopically. Rheological master curves of frequency sweeps of the extrudates exhibited a strong dependence on the API chemistry and revealed a rank ordering of the relative strength of API-copovidone interactions, in the order of posaconazole > nifedipine > clotrimazole. NMR data provided the means to precisely map the API-polymer interaction pattern and identify the specific sites of interaction from a molecular perspective. Finally, the impact of API-polymer interactions on the physical stability of the resultant extrudates was studied.
Conclusion
Qualitative and quantitative evaluation of the relative strength of the API-polymer interaction was successfully accomplished by utilizing combined rheology and NMR.

Graphical Abstract











Similar content being viewed by others
References
Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Kumar Battu S, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33:909–26.
Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Ind Pharm. 2007;33:1043–57.
Brown C, DiNunzio J, Eglesia M, Forster S, Lamm M, Lowinger M, et al. Hot-melt extrusion for solid dispersions: composition and design considerations. Amorphous solid dispersions: Springer; 2014. p. 197–230.
Rumondor AC, Ivanisevic I, Bates S, Alonzo DE, Taylor LS. Evaluation of drug-polymer miscibility in amorphous solid dispersion systems. Pharm Res. 2009;26:2523–34.
Song Y, Yang X, Chen X, Nie H, Byrn S, Lubach JW. Investigation of drug–excipient interactions in Lapatinib amorphous solid dispersions using solid-state NMR spectroscopy. Mol Pharm. 2015;12:857–66.
Marsac PJ, Li T, Taylor LS. Estimation of drug–polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2009;26:139–51.
Nie H, Mo H, Zhang M, Song Y, Fang K, Taylor LS, et al. Investigating the interaction pattern and structural elements of a drug–polymer complex at the molecular level. Mol Pharm. 2015;12:2459–68.
Nie H, Su Y, Zhang M, Song Y, Leone A, Taylor LS, et al. Solid-state spectroscopic investigation of molecular interactions between Clofazimine and Hypromellose phthalate in amorphous solid dispersions. Mol Pharm. 2016;13:3964–75.
Liu H, Zhang X, Suwardie H, Wang P, Gogos CG. Miscibility studies of indomethacin and Eudragit® E PO by thermal, rheological, and spectroscopic analysis. J Pharm Sci. 2012;101:2204–12.
Yang M, Wang P, Suwardie H, Gogos C. Determination of acetaminophen's solubility in poly (ethylene oxide) by rheological, thermal and microscopic methods. Int J Pharm. 2011;403:83–9.
Maniruzzaman M, Morgan DJ, Mendham AP, Pang J, Snowden MJ, Douroumis D. Drug–polymer intermolecular interactions in hot-melt extruded solid dispersions. Int J Pharm. 2013;443:199–208.
Punčochová K, Heng JYY, Beránek J, Štěpánek F. Investigation of drug–polymer interaction in solid dispersions by vapour sorption methods. Int J Pharm. 2014;469:159–67.
Sarode AL, Sandhu H, Shah N, Malick W, Zia H. Hot melt extrusion (HME) for amorphous solid dispersions: predictive tools for processing and impact of drug–polymer interactions on supersaturation. Eur J Pharm Sci. 2013;48:371–84.
Huang J, Wigent RJ, Schwartz JB. Drug–polymer interaction and its significance on the physical stability of nifedipine amorphous dispersion in microparticles of an ammonio methacrylate copolymer and ethylcellulose binary blend. J Pharm Sci. 2008;97:251–62.
Tian Y, Booth J, Meehan E, Jones DS, Li S, Andrews GP. Construction of drug–polymer thermodynamic phase diagrams using Flory–Huggins interaction theory: identifying the relevance of temperature and drug weight fraction to phase separation within solid dispersions. Mol Pharm. 2012;10:236–48.
Jhaand PK, Larson RG. Assessing the efficiency of polymeric excipients by atomistic molecular dynamics simulations. Mol Pharm. 2014;11:1676–86.
Lu X, Huang C, Lowinger MB, Yang F, Xu W, Brown CD, et al. Molecular interactions in Posaconazole amorphous solid dispersions from two-dimensional solid-state NMR spectroscopy. Mol Pharm. 2019;16:2579–89.
Meng F, Jing Z, Ferreira R, Ren P, Zhang F. Investigating the association mechanism between Rafoxanide and Povidone. Langmuir. 2018;34:13971–8.
Overhoff KA, Moreno A, Miller DA, Johnston KP, Williams RO. Solid dispersions of itraconazole and enteric polymers made by ultra-rapid freezing. Int J Pharm. 2007;336:122–32.
Bochmann ES, Neumann D, Gryczke A, Wagner KG. Micro-scale prediction method for API-solubility in polymeric matrices and process model for forming amorphous solid dispersion by hot-melt extrusion. Eur J Pharm Biopharm. 2016;107:40–8.
Saerens L, Dierickx L, Quinten T, Adriaensens P, Carleer R, Vervaet C, et al. In-line NIR spectroscopy for the understanding of polymer–drug interaction during pharmaceutical hot-melt extrusion. Eur J Pharm Biopharm. 2012;81:230–7.
Ewing AV, Clarke GS, Kazarian SG. Stability of indomethacin with relevance to the release from amorphous solid dispersions studied with ATR-FTIR spectroscopic imaging. Eur J Pharm Sci. 2014;60:64–71.
Saerens L, Dierickx L, Lenain B, Vervaet C, Remon JP, De Beer T. Raman spectroscopy for the in-line polymer–drug quantification and solid state characterization during a pharmaceutical hot-melt extrusion process. Eur J Pharm Biopharm. 2011;77:158–63.
Newman A, Engers D, Bates S, Ivanisevic I, Kelly RC, Zografi G. Characterization of amorphous API: polymer mixtures using X-ray powder diffraction. J Pharm Sci. 2008;97:4840–56.
K. Kothari, V. Ragoonanan, and R. Suryanarayanan. The role of drug–polymer hydrogen bonding interactions on the molecular mobility and physical stability of nifedipine solid dispersions. (2014).
Meng F, Trivino A, Prasad D, Chauhan H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur J Pharm Sci. 2015;71:12–24.
Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug–polymer miscibility and solubility. Pharm Res. 2006;23:2417–26.
Ghebremeskel AN, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: selection of polymer–surfactant combinations using solubility parameters and testing the processability. Int J Pharm. 2007;328:119–29.
Chan S-Y, Qi S, Craig DQ. An investigation into the influence of drug–polymer interactions on the miscibility, processability and structure of polyvinylpyrrolidone-based hot melt extrusion formulations. Int J Pharm. 2015;496:95–106.
Ivanisevic I, Bates S, Chen P. Novel methods for the assessment of miscibility of amorphous drug-polymer dispersions. J Pharm Sci. 2009;98:3373–86.
Meng F, Dave V, Chauhan H. Qualitative and quantitative methods to determine miscibility in amorphous drug–polymer systems. Eur J Pharm Sci. 2015;77:106–11.
Lamm MS, Simpson A, McNevin M, Frankenfeld C, Nay R, Variankaval N. Probing the effect of drug loading and humidity on the mechanical properties of solid dispersions with nanoindentation: antiplasticization of a polymer by a drug molecule. Mol Pharm. 2012;9:3396–402.
Van Renterghem J, Vervaet C, De Beer T. Rheological characterization of molten polymer-drug dispersions as a predictive tool for pharmaceutical hot-melt extrusion processability. Pharmaceutical Research. 2017:1–10.
Bochmann ES, Üstüner EE, Gryczke A, Wagner KG. Predicting melt rheology for hot-melt extrusion by means of a simple Tg-measurement. Eur J Pharm Biopharm. 2017;119:47–55.
Aho J, Boetker JP, Baldursdottir S, Rantanen J. Rheology as a tool for evaluation of melt processability of innovative dosage forms. Int J Pharm. 2015;494:623–42.
Yang F, Su Y, Zhu L, Brown CD, Rosen LA, Rosenberg KJ. Rheological and solid-state NMR assessments of copovidone/clotrimazole model solid dispersions. Int J Pharm. 2016;500:20–31.
Yang F, Su Y, Zhang J, DiNunzio J, Leone A, Huang C, et al. Rheology guided rational selection of processing temperature to prepare Copovidone–Nifedipine amorphous solid dispersions via hot melt extrusion (HME). Mol Pharm. 2016;13:3494–505.
Gupta SS, Parikh T, Meena AK, Mahajan N, Vitez I, Serajuddin AT. Effect of carbamazepine on viscoelastic properties and hot melt extrudability of Soluplus®. Int J Pharm. 2015;478:232–9.
Zecevicand DE, Wagner KG. Rational development of solid dispersions via hot-melt extrusion using screening, material characterization, and numeric simulation tools. J Pharm Sci. 2013;102:2297–310.
Suwardie H, Wang P, Todd DB, Panchal V, Yang M, Gogos CG. Rheological study of the mixture of acetaminophen and polyethylene oxide for hot-melt extrusion application. Eur J Pharm Biopharm. 2011;78:506–12.
Bhardwaj SP, Arora KK, Kwong E, Templeton A, Clas S-D, Suryanarayanan R. Mechanism of amorphous itraconazole stabilization in polymer solid dispersions: role of molecular mobility. Mol Pharm. 2014;11:4228–37.
Leonardi F, Derail C, Marin G. Some applications of molecular rheology: polymer formulation and molecular design. J Non-Newtonian Fluid Mech. 2005;128:50–61.
Janeschitz-Kriegl H. Polymer melt rheology and flow birefringence: Springer Science & Business Media; 2012.
Paudel A, Geppi M, Van den Mooter G. Structural and dynamic properties of amorphous solid dispersions: the role of solid-state nuclear magnetic resonance spectroscopy and relaxometry. J Pharm Sci. 2014;103:2635–62.
Berendt RT, Sperger DM, Munson EJ, Isbester PK. Solid-state NMR spectroscopy in pharmaceutical research and analysis. TrAC Trends Anal Chem. 2006;25:977–84.
Urbanova M, Brus J, Sedenkova I, Policianova O, Kobera L. Characterization of solid polymer dispersions of active pharmaceutical ingredients by 19 F MAS NMR and factor analysis. Spectrochim Acta A Mol Biomol Spectrosc. 2013;100:59–66.
Rossini AJ, Widdifield CM, Zagdoun A, Lelli M, Schwarzwälder M, Copéret C, et al. Dynamic nuclear polarization enhanced NMR spectroscopy for pharmaceutical formulations. J Am Chem Soc. 2014;136:2324–34.
Tatton AS, Pham TN, Vogt FG, Iuga D, Edwards AJ, Brown SP. Probing hydrogen bonding in cocrystals and amorphous dispersions using 14N–1H HMQC solid-state NMR. Mol Pharm. 2013;10:999–1007.
Školáková T, Souchová L, Patera J, Pultar M, Školáková A, Zámostný P. Prediction of drug-polymer interactions in binary mixtures using energy balance supported by inverse gas chromatography. Eur J Pharm Sci. 2019;130:247–59.
Adrjanowicz K, Kaminski K, Wlodarczyk P, Grzybowska K, Tarnacka M, Zakowiecki D, et al. Molecular dynamics of the supercooled pharmaceutical agent posaconazole studied via differential scanning calorimetry and dielectric and mechanical spectroscopies. Mol Pharm. 2013;10:3934–45.
Tsakiridou G, Reppas C, Kuentz M, Kalantzi L. A novel rheological method to assess drug-polymer interactions regarding miscibility and crystallization of drug in amorphous solid dispersions for oral drug delivery. Pharmaceutics. 2019;11:625.
Van Gurp M, Palmen J. Time-temperature superposition for polymeric blends. Rheol Bull. 1998;67:5–8.
Juster H, Distlbacher T, Steinbichler G. Viscosity analysis of a polymer-based drug delivery system using open-source CFD methods and high-pressure capillary Rheometry. Int Polym Process. 2014;29:570–8.
Graessley WW. The entanglement concept in polymer rheology. The entanglement concept in polymer rheology: Springer; 1974. p. 1–179.
Liu H, Wang P, Zhang X, Shen F, Gogos CG. Effects of extrusion process parameters on the dissolution behavior of indomethacin in Eudragit® E PO solid dispersions. Int J Pharm. 2010;383:161–9.
Treffer D, Troiss A, Khinast J. A novel tool to standardize rheology testing of molten polymers for pharmaceutical applications. Int J Pharm. 2015;495:474–81.
Balogh A, Drávavölgyi G, Faragó K, Farkas A, Vigh T, Sóti PL, et al. Plasticized drug-loaded melt electrospun polymer mats: characterization, thermal degradation, and release kinetics. J Pharm Sci. 2014;103:1278–87.
Prudic A, Ji Y, Sadowski G. Thermodynamic phase behavior of API/polymer solid dispersions. Mol Pharm. 2014;11:2294–304.
Qian F, Huang J, Hussain MA. Drug–polymer solubility and miscibility: stability consideration and practical challenges in amorphous solid dispersion development. J Pharm Sci. 2010;99:2941–7.
Patil H, Tiwari RV, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2016;17:20–42.
Teja SB, Patil SP, Shete G, Patel S, Bansal AK. Drug-excipient behavior in polymeric amorphous solid dispersions. J Excip Food Chem. 2016;4.
Songand H, Shin H-S. The antifungal drug clotrimazole. Acta Crystallogr Sect C: Cryst Struct Commun. 1998;54:1675–7.
Ehmann HM, Zimmer A, Roblegg E, Werzer O. Morphologies in solvent-annealed clotrimazole thin films explained by Hansen-solubility parameters. Cryst Growth Des. 2014;14:1386–91.
Das S, Ng WK, Tan RB. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci. 2012;47:139–51.
Gunn E, Guzei IA, Cai T, Yu L. Polymorphism of nifedipine: crystal structure and reversible transition of the metastable β polymorph. Cryst Growth Des. 2012;12:2037–43.
Hirayama F, Wang Z, Uekama K. Effect of 2-hydroxypropyl-β-cyclodextrin on crystallization and polymorphic transition of nifedipine in solid state. Pharm Res. 1994;11:1766–70.
Grooff D, Liebenberg W, De Villiers MM. Preparation and transformation of true nifedipine polymorphs: investigated with differential scanning calorimetry and X-ray diffraction pattern fitting methods. J Pharm Sci. 2011;100:1944–57.
Grooff D, De Villiers M, Liebenberg W. Thermal methods for evaluating polymorphic transitions in nifedipine. Thermochim Acta. 2007;454:33–42.
Tang P, Ma X, Wu D, Li S, Xu K, Tang B, et al. Posaconazole/hydroxypropyl-β-cyclodextrin host–guest system: improving dissolution while maintaining antifungal activity. Carbohydr Polym. 2016;142:16–23.
Fuleand R, Amin P. Hot melt extruded amorphous solid dispersion of posaconazole with improved bioavailability: investigating drug-polymer miscibility with advanced characterisation. Biomed Res Int. 2014;2014.
Qian F, Huang J, Zhu Q, Haddadin R, Gawel J, Garmise R, et al. Is a distinctive single T g a reliable indicator for the homogeneity of amorphous solid dispersion? Int J Pharm. 2010;395:232–5.
Baird JA, Van Eerdenbrugh B, Taylor LS. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J Pharm Sci. 2010;99:3787–806.
Ferry JD. Viscoelastic properties of polymers: John Wiley & Sons; 1980.
Crossley RJ, Schubel PJ, De Focatiis DSA. Time–temperature equivalence in the tack and dynamic stiffness of polymer prepreg and its application to automated composites manufacturing. Compos A: Appl Sci Manuf. 2013;52:126–33.
Van Krevelen DW, Te Nijenhuis K. Properties of polymers: their correlation with chemical structure; their numerical estimation and prediction from additive group contributions: Elsevier; 2009.
Cai H, Ait-Kadi A, Brisson J. Dynamic rheological analysis of a miscible blend showing strong interactions. Polymer. 2003;44:1481–9.
Sopade P, Halley P, Bhandari B, D’arcy B, Doebler C, Caffin N. Application of the Williams–Landel–Ferry model to the viscosity–temperature relationship of Australian honeys. J Food Eng. 2003;56:67–75.
Lomellini P. Williams-Landel-Ferry versus Arrhenius behaviour: polystyrene melt viscoelasticity revised. Polymer. 1992;33:4983–9.
Angell C. Why C 1= 16–17 in the WLF equation is physical—and the fragility of polymers. Polymer. 1997;38:6261–6.
Jacksonand W, Caldwell J. Antiplasticization. II Characteristics of antiplasticizers. J Appl Polym Sci. 1967;11:211–26.
Ahoand J, Syrjälä S. On the measurement and modeling of viscosity of polymers at low temperatures. Polym Test. 2008;27:35–40.
Kothari K, Ragoonanan V, Suryanarayanan R. The role of drug–polymer hydrogen bonding interactions on the molecular mobility and physical stability of Nifedipine solid dispersions. Mol Pharm. 2015;12:162–70.
Marsac PJ, Rumondor AC, Nivens DE, Kestur US, Stanciu L, Taylor LS. Effect of temperature and moisture on the miscibility of amorphous dispersions of felodipine and poly (vinyl pyrrolidone). J Pharm Sci. 2010;99:169–85.
Hilton BD, Feng W, Martin GE. Assignment of the 15N resonances of the antifungal agent posaconazole. J Heterocyclic Chem. 2011;48:948–51.
Hiltonand BD, Martin GE. The impact of long-range 1H-15N Heteronuclear shift correlation data on computer-assisted structure elucidation: posaconazole. J Heterocyclic Chem. 2012;49:526–32.
Martin GE. Posaconazole: application of HSQC-ADEQUATE from general indirect covariance processing. J Heterocyclic Chem. 2012;49:716–20.
Mistry P, Mohapatra S, Gopinath T, Vogt FG, Suryanarayanan R. Role of the strength of drug–polymer interactions on the molecular mobility and crystallization inhibition in ketoconazole solid dispersions. Mol Pharm. 2015;12:3339–50.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors are grateful to MRL for the grant and support of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 322 kb)
Rights and permissions
About this article
Cite this article
Yang, F., Su, Y., Small, J. et al. Probing the Molecular-Level Interactions in an Active Pharmaceutical Ingredient (API) - Polymer Dispersion and the Resulting Impact on Drug Product Formulation. Pharm Res 37, 94 (2020). https://doi.org/10.1007/s11095-020-02813-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02813-z