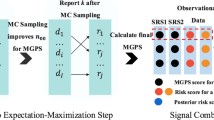
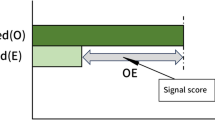
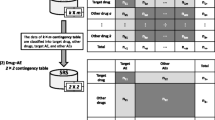
Abstract
Purpose
Adverse events (AEs) caused by polypharmacy have recently become a clinical problem, and it is important to monitor the safety profile of drug-drug interactions (DDIs). Mining signals using the spontaneous reporting systems is a very effective method for single drug-induced AE monitoring as well as early detection of DDIs. The objective of this study was to compare signal detection algorithms for DDIs based on frequency statistical models.
Methods
Five frequency statistical models: the Ω shrinkage measure, additive (risk difference), multiplicative (risk ratio), combination risk ratio, and chi-square statistics models were compared using the Japanese Adverse Drug Event Report (JADER) database as the spontaneous reporting system in Japan. The drugs targeted for the survey are all registered and classified as “suspect drugs” in JADER, and the AEs targeted for this study were the same as those in a previous study on Stevens-Johnson syndrome (SJS).
Results
Of 3924 pairs that reported SJS, the number of signals detected by the Ω shrinkage measure, additive, multiplicative, combination risk ratio, and chi-square statistics models was 712, 3298, 2252, 739, and 1289 pairs, respectively. Among the five models, the Ω shrinkage measure model showed the most conservative signal detection tendency.
Conclusion
Specifically, caution should be exercised when the number of reports is low because results differ depending on the statistical models. This study will contribute to the selection of appropriate statistical models to detect signals of potential DDIs.




Similar content being viewed by others
Abbreviations
- AE:
-
adverse events
- CI:
-
Confidence interval
- csv:
-
comma-separated values
- DDI:
-
drug-drug interaction
- FAERS:
-
Food and Drug Administration Adverse Events Reporting System
- IC:
-
information component
- JADER:
-
Japanese Adverse Drug Event Report database
- MedDRA /J:
-
Medical Dictionary for Regulatory Activities Japanese version
- MGPS:
-
multi gamma–Poisson shrinker
- PT:
-
preferred term
- PRR:
-
proportional reporting ratio
- RGPS:
-
regression-adjusted gamma–Poisson shrinkage
- SJS:
-
Stevens-Johnson syndrome,
References
Norén GN, Bate A, Orre R, Edwards IR. Extending the methods used to screen the WHO drug safety database towards analysis of complex associations and improved accuracy for rare events. Stat Med. 2006;25:3740–57. https://doi.org/10.1002/sim.2473.
Almenoff JS, DuMouchel W, Kindman LA, Yang X, Ram D. Disproportionality analysis using empirical Bayes data mining: a tool for the evaluation of drug interactions in the post-marketing setting. Pharmacoepidemiol Drug Saf. 2003;12:517–21. https://doi.org/10.1002/pds.885.
Yang X, Fram D. Using disproportionality analysis as a tool to explore drug-drug interavtions in AERS database. Pharmacoepidemiol Drug Saf. 2004;13:S247.
DuMouchel W, Harpaz R. (2012). Regression-adjusted GPS algorithm (RGPS). An Oracle White Paper November.
Vilar S, Friedman C, Hripcsak G. Detection of drug-drug interactions through data mining studies using clinical sources, scientific literature and social media. Brief Bioinform. 2018;19:863–77. https://doi.org/10.1093/bib/bbx010.
Noguchi Y, Tachi T, Teramachi H. Review of statistical methodologies for detecting drug-drug interactions using spontaneous reporting systems. Front Pharmacol. 2019;10:1319. https://doi.org/10.3389/fphar.2019.01319.
Norén GN, Sundberg R, Bate A, Edwards IR. A statistical methodology for drug-drug interaction surveillance. Stat Med. 2008;27:3057–70. https://doi.org/10.1002/sim.3247.
Thakrar BT, Grundschober SB, Doessegger L. Detecting signals of drug-drug interactions in a spontaneous reports database. Br J Clin Pharmacol. 2007;64:489–95. https://doi.org/10.1111/j.1365-2125.2007.02900.x.
Susuta Y, Takahashi Y. Safety risk evaluation methodology in detecting the medicine concomitant use risk which might cause critical drug rash. Jpn J Pharmacoepidemiol. 2014;19:39–49. https://doi.org/10.3820/jjpe.19.39.
Gosho M, Maruo K, Tada K, Hirakawa A. Utilization of chi-square statistics for screening adverse drug-drug interactions in spontaneous reporting systems. Eur J Clin Pharmacol. 2017;73:779–86. https://doi.org/10.1007/s00228-017-2233-3.
Van Puijenbroek EP, Bate A, Leufkens HGM, Lindquist M, Egberts OR, ACG. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11:3–10.
Gould AL. Practical pharmacovigilance analysis strategies. Pharmacoepidemiol Drug Saf. 2003;12:559–74.
Kubota K, Koide D, Hirai T. Comparison of data mining methodologies using Japanese spontaneous reports. Pharmacoepidemiol Drug Saf. 2004;13:387–94. https://doi.org/10.1002/pds.964.
Sakaeda T, Kadoyama K, Minami K, Okuno Y. Commonality of drug-associated adverse events detected by 4 commonly used data mining algorithms. Int J Med Sci. 2014;11:461–5.
Pham M, Cheng F, Ramachandran K. A comparison study of algorithms to detect drug-adverse event associations: Frequentist, Bayesian, and machine-learning approaches. Drug Saf. 2019;42:743–50. https://doi.org/10.1007/s40264-018-00792-0.
Feinstein AR. Evaluating concordances. In: Principles of medical statistics. Chapman & Hall/CRC: Boca Raton; 2002. p. 407–36.
Iwasaki M, Yoshida K. Statistical Inference for the Occurrence Probability of Rare Events -Rule of Three and Related Topics- Jpn Journal Biomet. 2005;26:53–63. https://doi.org/10.5691/jjb.26.53.
Ponzo MG, Miliszewski M, Kirchhof MG, Keown PA, Dutz JP. HLA-B*58:01 genotyping to prevent cases of DRESS and SJS/TEN in east Asians treated with allopurinol-a Canadian missed opportunity. J Cutan Med Surg. 2019;23:595–601. https://doi.org/10.1177/1203475419867599.
Comparin C, Hans Filho G, Takita LC, Costa Nde C, Nascimento RA, Nanni Lde O. Treatment of toxic epidermal necrolysis with intravenous immunoglobulin: a series of three cases. An Bras Dermatol. 2012;87(3):477–81. https://doi.org/10.1590/s0365-05962012000300022.
Nagashima T, Shirakawa H, Nakagawa T, Kaneko S. Prevention of antipsychotic-induced hyperglycaemia by vitamin D: a data mining prediction followed by experimental exploration of the molecular mechanism. Sci Rep. 2016;6:26375. https://doi.org/10.1038/srep26375.
Uno T, Wada K, Hosomi K, Matsuda S, Ikura MM, Takenaka H, et al. Drug interactions between tacrolimus and clotrimazole troche: a data mining approach followed by a pharmacokinetic study. Eur J Clin Pharmacol. 2020;76:117–25. https://doi.org/10.1007/s00228-019-02770-6.
Caster O, Aoki Y, Gattepaille LM, Grundmark B. Disproportionality analysis for Pharmacovigilance signal detection in small databases or subsets: recommendations for limiting false-positive associations. Drug Saf. 2020:1–9. https://doi.org/10.1007/s40264-020-00911-w.
Acknowledgments and Disclosures
Yoshihiro Noguchi, Tomoya Tachi and Hitomi Teramachi have no conflicts of interest that are directly relevant to the content of this study.
Funding
This study was supported by JSPS KAKENHI Grant Number 19 K20731.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noguchi, Y., Tachi, T. & Teramachi, H. Comparison of Signal Detection Algorithms Based on Frequency Statistical Model for Drug-Drug Interaction Using Spontaneous Reporting Systems. Pharm Res 37, 86 (2020). https://doi.org/10.1007/s11095-020-02801-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02801-3