Abstract
Purpose
Combination of PCI and chemotherapy represents a promising strategy for combating drug resistance of cancer. However, poor solubility of photosensitizers and unselectively released drugs at unwanted sites significantly impaired the treatment efficacy. Therefore, in the present study, we aimed to develop a nano-platform which could efficiently co-entrapping photosensitizers and chemotherapeutics for active targeting therapy of drug resistant cancers.
Methods
Two pro-drugs were respectively developed by covalently linking the Ce6 with each other via the GSH-sensitive linkage and the PTX with mPEG-PLA-COOH through the ROS sensitive-linker. The dual-responsive nanoparticles (PNP-Ce6) was developed by emulsion/solvent evaporation method and further modified with tLyp-1 peptides. Physicochemical properties of nanoparticles were determined by the TEM and DLC. Cellular uptake assay was investigated with the Ce6 acting as the fluorescent probe and cell growth was studied by the MTT experiment. In vivo tumor targeting and anti-tumor assay was investigated on the colorectal cancer-bearing mice.
Results
The developed tPNP-Ce6 were stable enough under the normal physiological conditions. However, free Ce6 and PTX were completely released when exposed the tPNP-Ce6 to the redox environment. Excellent tumor-targeting drug delivery was achieved by the tPNP-Ce6, which in turn resulted in satisfactory anit-tumor effect. Of great importance, super inhibition effect on tumor progress was achieved by the combination therapy when compared with the group only received with chemotherapy..
Conclusion
The results obtained in the present study indicated that the developed tPNP-Ce6 may have great potential in enhancing the therapeutic efficacy of drug-resistant colorectal cancer.

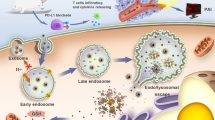
Left: Targeting delivery of drug to tumor site by the tumor recognizable and dual-responsive nanoparticles and penetrating into tumor inner via the mediation of irradiation. Right: Nanoparticle distribution within tumor tissues with green represents the blood vessels stained with CD31, blue signal represents the cell nuclei stained with DAPI and red shows fluorescence of Ce6 as the indicator of the nanoparticles.










Similar content being viewed by others
Abbreviations
- Ce6:
-
Chlorin e6
- DAPI 40,6:
-
Diamidino-2-phenylindole
- DCC 1, 3:
-
Dicyclohexyl carbodiimide
- DLC:
-
Dynamic light scattering detector
- DMAP:
-
4-Dimethylaminopyridine
- DMSO:
-
Dimethyl sulfoxide
- EE:
-
Encapsulation efficacy
- GSH:
-
γ-glutamylcysteinyl-glycine
- HCT-15:
-
Human colorectal cancer cells
- HPLC:
-
High performance liquid chromatography
- LC:
-
Loading capacity
- M-β-CD:
-
methyl-β-cyclodextrin
- NRP:
-
1 neuropilin-1
- PCI:
-
Photodynamic internalization
- PDT:
-
Photodynamic therapy
- P-gp:
-
P-glycoprotein
- PS:
-
Photosensitizers
- PTX:
-
Paclitaxel
- ROS:
-
Reactive oxygen species
- SOG:
-
Singlet oxygen generation
- TEM:
-
Transmission electron microscope
- TK:
-
Thioketal groups
- -SS-mal:
-
Maleimide thioether
References
Patel NR, Rathi A, Mongayt D, Torchilin VP. Reversal of multidrug resistance by co-delivery of tariquidar (XR9576) and paclitaxel using long-circulating liposomes. Int J Pharm. 2011;16(1):296–9.
Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6(4):268–86.
Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323(5922):1718–22.
Chen Y, Zhang W, Gu J, Ren Q, Fan Z, Zhong W, et al. Enhanced antitumor efficacy by methotrexate conjugated Pluronic mixed micelles against KBv multidrug resistant cancer. Int J Pharm. 2013;452(1–2):421–33.
Jiang D, Gao X, Kang T, Feng X, Yao J, Yang M, et al. Actively targeting D-α-tocopheryl polyethylene glycol 1000 succinate-poly(lactic acid) nanoparticles as vesicles for chemo-photodynamic combination therapy of doxorubicin-resistant breast cancer. Nanoscale. 2016;8(5):3100–18.
Lage H. MDR1/P-glycoprotein (ABCB1) as target for RNA interference-mediated reversal of multidrug resistance. Curr Drug Targets. 2006;7(7):813–21.
Patil Y, Sadhukha T, Ma L, Panyam J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release. 2009;136(1):21–9.
Kunjachan S, Rychlik B, Storm G, Kiessling F, Lammers T. Multidrug resistance: physiological principles and nanomedical solutions. Adv Drug Deliv Rev. 2013;65(13–14):1852–65.
Zhang J, Du Z, Pan S, Shi M, Li J, Yang C, et al. Overcoming multidrug resistance by Codelivery of MDR1-targeting siRNA and doxorubicin using EphA10-mediated pH-sensitive Lipoplexes: in vitro and in vivo evaluation. ACS Appl Mater Interfaces. 2018;10(25):21590–600.
Bai F, Wang C, Lu Q, Zhao M, Ban FQ, Yu DH, et al. Nanoparticle-mediated drug delivery to tumor neovasculature to combat P-gp expressing multidrug resistant cancer. Biomaterials. 2013;34:6163–74.
Hubensack M, Müller C, Höcherl P, Fellner S, Spruss T, Bernhardt G, et al. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. J Cancer Res Clin Oncol. 2008;134(5):597–607.
McDevitt CA, Callaghan R. How can we best use structural information on P-glycoprotein to design inhibitors? Pharmacol Ther. 2007;113(2):429–41.
Berg K, Dietze A, Kaalhus O, Høgset A. Site-specific drug delivery by photochemical internalization enhances the antitumor effect of bleomycin. Clin Cancer Res. 2005;11(23):8476–85.
Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B. 2009;96(1):1–8.
Sadaksharam J, Nayaki KP, Selvam NP. Treatment of oral lichen planus with methylene blue mediated photodynamic therapy-a clinical study. Photodermatol Photoimmunol Photomed. 2012;28(2):97–101.
Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, et al. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38(5):468–81.
Lau JT, Lo PC, Fong WP, Ng DK. A zinc(II) phthalocyanine conjugated with an oxaliplatin derivative for dual chemo- and photodynamic therapy. J Med Chem. 2012;55:5446–54.
Lee CS, Park W, Park SJ, Na K. Endolysosomal environment-responsive photodynamic nanocarrier to enhance cytosolic drug delivery via photosensitizer-mediated membrane disruption. Biomaterials. 2013;34(36):9227–36.
Bostad M, Olsen CE, Peng Q, Berg K, Høgset A, Selbo PK. Light-controlled endosomal escape of the novel CD133-targeting immunotoxin AC133-saporin by photochemical internalization-a minimally invasive cancer stem cell-targeting strategy. J Control Release. 2015;206:37–48.
Lu HL, Syu WJ, Nishiyama N, Kataoka K, Lai PS. Dendrimer phthalocyanine-encapsulated polymeric micelle-mediated photochemical internalization extends the efficacy of photodynamic therapy and overcomes drug-resistance in vivo. J Control Release. 2011;155(3):458–64.
Zheng X, Xing D, Zhou F, Wu B, Chen WR. Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol Pharm. 2011;8(2):447–56.
Almutairi A, Guillaudeu SJ, Berezin MY, Achilefu S, Fréchet JM. Biodegradable pH-sensing dendritic nanoprobes for near-infrared fluorescence lifetime and intensity imaging. J Am Chem Soc. 2008;130(2):444–5.
Miki K, Kimura A, Oride K, Kuramochi Y, Matsuoka H, Harada H, et al. High-contrast fluorescence imaging of tumors in vivo using nanoparticles of amphiphilic brush-like copolymers produced by ROMP. Angew Chem Int Ed Engl. 2011;50(29):6567–70.
Luo S, Zhang E, Su Y, Cheng T, Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32(29):7127–38.
Roth L, Agemy L, Kotamraju VR, Braun G, Teesalu T, Sugahara KN, et al. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene. 2012;31(33):3754–63.
Yang S, Gao H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res. 2017;126:97–108.
Shim MS, Xia Y. A reactive oxygen species (ROS)-responsive polymer for safe, efficient, and targeted gene delivery in Cancer cells. Angew Chem Int Ed Engl. 2013;52(27):6926–9.
Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, et al. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16(6):510–20.
He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90(4):739–51.
Ruan S, Yuan M, Zhang L, Hu G, Chen J, Cun X, et al. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials. 2015;37:425–35.
Liu R, Xiao W, Hu C, Xie R, Gao H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J Control Release. 2018;278:127–39.
Park H, Park W, Na K. Doxorubicin loaded singlet-oxygen producible polymeric micelle based on chlorine e6 conjugated pluronic F127 for overcoming drug resistance in cancer. Biomaterials. 2014;35(27):7963–9.
Gao H. Perspectives on dual targeting delivery systems for brain tumors. J NeuroImmune Pharmacol. 2017;12(1):6–16.
Liu R, Hu C, Yang Y, Zhang J, Gao H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm Sin B. 2019;9(2):410–20.
Xiao W, Xiong J, Zhang S, Xiong Y, Zhang H, Gao H. Influence of ligands property and particle size of gold nanoparticles on the protein adsorption and corresponding targeting ability. Int J Pharm. 2018;538(1–2):105–11.
Xiao W, Gao H. The impact of protein corona on the behavior and targeting capability of nanoparticle-based delivery system. Int J Pharm. 2018;552(1–2):328–39.
Acknowledgments and Disclosures
This work was supported by Sichuan University Talent Introduction Start-up Fund (Jingyuan Xiong) and also the Public Health and Preventive Medicine Provincal Experiment Teaching Center at Sichuan University, as well as the Food Safety Monitoring and Risk Assessment Key Laboratory of Sichuan Province.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, Y., Shan, S., Li, C. et al. Application of the Tumor Site Recognizable and Dual-Responsive Nanoparticles for Combinational Treatment of the Drug-Resistant Colorectal Cancer. Pharm Res 37, 72 (2020). https://doi.org/10.1007/s11095-020-02791-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02791-2