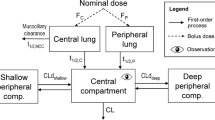
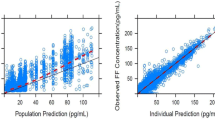
Abstract
Purpose
Inhaled delivery of pirfenidone to the lungs of patients with idiopathic pulmonary fibrosis holds promise to eliminate oral-observed side effects while enhancing efficacy. This study aimed to comprehensively describe the pulmonary pharmacokinetics of inhaled aerosol pirfenidone in healthy adult sheep. Methods: Pirfenidone concentrations were evaluated in plasma, lung-derived lymph and epithelial lining fluid (ELF) with data subjected to non-compartmental pharmacokinetic analysis. Results: Compartmental pharmacokinetic evaluation indicated that a 49 mg lung-deposited dose delivered an ELF Cmax of 62 ± 23 mg/L, and plasma Cmax of 3.1 ± 1.7 mg/L. Further analysis revealed that plasma pirfenidone reached Tmax faster and at higher concentrations than in lymph. These results suggested inhaled pirfenidone was cleared from the alveolar interstitium via blood faster than the drug could equilibrate between the lung interstitial fluid and lung lymphatics. However, the data also suggested that a ‘reservoir’ of pirfenidone feeds into lung lymph at later time points (after it has largely been cleared from plasma), prolonging lung lymphatic exposure.
Conclusions
This study indicates inhaled pirfenidone efficiently deposits in ELF and is cleared from the lungs by initial absorption into plasma, followed by later equilibrium with lung interstitial and lymph fluid.




Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the plasma concentration vs time curve
- BALF:
-
Bronchoalveolar lavage fluid
- Cmax:
-
Maximum concentration
- ELF:
-
Epithelial lining fluid
- Fabs:
-
Fraction of drug absorbed
- IC50:
-
50% inhibitory concentration
- Tmax:
-
Time to maximum concentration
- IPF:
-
Idiopathic pulmonary fibrosis
References
American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–64.
Spagnolo P, Del Giovane C, Luppi F, Cerri S, Balduzzi S, Walters EH, D'Amico R, Richeldi L. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010;9:CD003134.
Sakai N, Tager AM. Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta. 2013;1832(7):911–21.
Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20(120):85–97.
Committee for Medicinal Products Human Use. CHMP assessment report. Esbret. 2010. Procedure No EMEA/H/C/002154.
Rubino CM, Bhavnani SM, Ambrose PG, Forrest A, Loutit JS. Effect of food and antacids on the pharmacokinetics of pirfenidone in older healthy adults. Pulm Pharmacol Ther. 2009;22(4):279–85.
Surber MW, Poulin D, McInally K, Chapdelaine J, Gendron D, Tat V, Murphy J, Ayaub E, Kolb MRJ, Ask K. Inhaled Pirfenidone improves animal efficacy through superior pulmonary and vascular pharmacokinetics. Am J Resp Crit Care. 2014;189
Khoo JK, Montgomery AB, Otto KL, Surber M, Faggian J, Lickliter JD, Glaspole I. A randomized, double-blinded, placebo-controlled, dose-escalation phase 1 study of aerosolized Pirfenidone delivered via the PARI investigational eFlow nebulizer in volunteers and patients with idiopathic pulmonary fibrosis. J Aerosol Med Pulm Drug Deliv. 2019;
Ryan GM, Bischof RJ, Enkhbaatar P, McLeod VM, Chan LJ, Jones SA, Owen DJ, Porter CJ, Kaminskas LM. A comparison of the pharmacokinetics and pulmonary lymphatic exposure of a generation 4 PEGylated Dendrimer following intravenous and aerosol administration to rats and sheep. Pharm Res. 2016;33(2):510–25.
Staub NC, Bland RD, Brigham KL, Demling R, Erdmann AJ 3rd, Woolverton WC. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975;19(5):315–20.
Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985). 1986;60(2):532–8.
Meeusen EN, Snibson KJ, Hirst SJ, Bischof RJ. Sheep as a model for the study and treatment of human asthma and other respiratory diseases. Drug Discov Today Dis Model. 2010;6:101–6.
Bulitta JB, Bingolbali A, Shin BS, Landersdorfer CB. Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J. 2011;13(2):201–11.
Bulitta JB, Landersdorfer CB. Performance and robustness of the Monte Carlo importance sampling algorithm using parallelized S-ADAPT for basic and complex mechanistic models. AAPS J. 2011;13(2):212–26.
Acred P, Brown DM, Clark BF, Mizen L. The distribution of antibacterial agents between plasma and lymph in the dog. Br J Pharmacol. 1970;39(2):439–46.
Anderson KE, Dencker H, Mardh PA, Akerlund M. Relationships between the concentrations of doxycycline in serum and in thoracic duct lymph after oral and intravenous administration in man. Chemotherapy. 1976;22(5):277–85.
Kaminskas LM, Kota J, McLeod VM, Kelly BD, Karellas P, Porter CJ. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J Control Release. 2009;140(2):108–16.
Trevaskis NL, Kaminskas LM, Porter CJ. From sewer to saviour - targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14(11):781–803.
Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 2004;1(4):338–44.
Kambouchner M, Bernaudin JF. Intralobular pulmonary lymphatic distribution in normal human lung using D2-40 antipodoplanin immunostaining. J Histochem Cytochem. 2009;57(7):643–8.
Schraufnagel DE. Lung lymphatic anatomy and correlates. Pathophysiology. 2010;17(4):337–43.
El-Chemaly S, Malide D, Zudaire E, Ikeda Y, Weinberg BA, Pacheco-Rodriguez G, Rosas IO, Aparicio M, Ren P, MacDonald SD, Wu HP, Nathan SD, Cuttitta F, McCoy JP, Gochuico BR, Moss J. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci U S A. 2009;106(10):3958–63.
Lara AR, Cosgrove GP, Janssen WJ, Huie TJ, Burnham EL, Heinz DE, Curran-Everett D, Sahin H, Schwarz MI, Cool CD, Groshong SD, Geraci MW, Tuder RM, Hyde DM, Henson PM. Increased lymphatic vessel length is associated with the fibroblast reticulum and disease severity in usual interstitial pneumonia and nonspecific interstitial pneumonia. Chest. 2012;142(6):1569–76.
Ebina M, Shibata N, Ohta H, Hisata S, Tamada T, Ono M, Okaya K, Kondo T, Nukiwa T. The disappearance of subpleural and interlobular lymphatics in idiopathic pulmonary fibrosis. Lymphat Res Biol. 2010;8(4):199–207.
Landersdorfer CB, Nguyen TH, Lieu LT, Nguyen G, Bischof RJ, Meeusen EN, Li J, Nation RL, McIntosh MP. Substantial Targeting Advantage Achieved by Pulmonary Administration of Colistin Methanesulfonate in a Large-Animal Model. Antimicrob Agents Chemother. 2017;61(1)
Boisson M, Jacobs M, Gregoire N, Gobin P, Marchand S, Couet W, Mimoz O. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother. 2014;58(12):7331–9.
Acknowledgments and Disclosures
This study was funded by Avalyn Pharma Inc. LMK is supported by an NHMRC CDF2.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaminskas, L.M., Landersdorfer, C.B., Bischof, R.J. et al. Aerosol Pirfenidone Pharmacokinetics after Inhaled Delivery in Sheep: a Viable Approach to Treating Idiopathic Pulmonary Fibrosis. Pharm Res 37, 3 (2020). https://doi.org/10.1007/s11095-019-2732-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2732-2