Abstract
Purpose
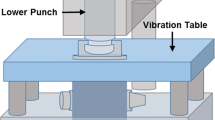
In the present study the influence and application of a newly developed external lower punch vibration system for an improved die filling on a running rotary tablet press was investigated.
Methods
Tablets were manufactured at different conditions (with and without vibration) and characterized regarding their direct compressibility and mechanical stability. Thus, two typical pharmaceutical binders for direct compression (Parmcel 102 and Tablettose® 80) were compared with two binders unsuitable for direct compression (Ceolus® KG1000 and GranuLac® 200). The powders were characterized by helium pycnometry, laser diffraction, scanning electron microscopy, and by determination of the powder flow. Furthermore, a novel technique to determine the occurrences of segregation within a tablet after manufacturing was introduced. For this purpose, a powder blend containing one spray-colored type of microcrystalline cellulose (Vivapur® 200) were prepared.
Results
It was shown that under application of externally applied lower punch vibration, the powder flow into the die increased and thus the die filling process was significantly improved. Hence, it was possible to manufacture tablets from powders, which are actually unsuitable for direct compression. In addition, the mechanical stability of the produced tablets was distinctly improved by application of lower punch vibration, whereby the occurrence of segregation was comparatively low.
Conclusion
In summary, lower punch vibration allows a more efficient die filling, whereby the powder flow as well as mechanical stability of the tablets are improved.









Similar content being viewed by others
Abbreviations
- Lac-1:
-
GranuLac® 200
- Lac-2:
-
Tablettose® 80
- MCC-1:
-
Ceolus® KG1000
- MCC-2:
-
Parmcel 102
- SF:
-
Solid fraction
References
Järvinen MA, Paaso J, Paavola M, Leiviskä K, Juuti M, Muzzio F, et al. Continuous direct tablet compression: effects of impeller rotation rate, total feed rate and drug content on the tablet properties and drug release. Drug Dev Ind Pharm. 2013;39:1802–8.
Hindiyeh M, Altalafha T, Al-Naerat M, Saidan H, Al-Salaymeh A, Sbeinati L, et al. Process modification of P’pharmaceutical tablet manufacturing operations: an eco-efficiency approach. Processes. 2018;6:1–8.
Goh HP, Heng PWS, Liew CV. Understanding effects of process parameters and forced feeding on die filling. Eur J Pharm Sci. 2018;122:105–15.
Sinka IC, Motazedian F, Cocks A, Pitt KG. The effect of processing parameters on pharmaceutical tablet properties. Powder Technol. 2009;189:276–84.
Augsburger LL, Hoag SW. Pharmaceutical dosage forms. 3rd ed. New York: Informa Healthcare; 2008.
Wu C-Y, Dihoru L, Cocks AC. The flow of powder into simple and stepped dies. Powder Technol. 2003;134:24–39.
Jackson S, Sinka IC, Cocks ACF. The effect of suction during die fill on a rotary tablet press. Eur J Pharm Biopharm. 2007;65:253–6.
Peeters E, de Beer T, Vervaet C, Remon J-P. Reduction of tablet weight variability by optimizing paddle speed in the forced feeder of a high-speed rotary tablet press. Drug Dev Ind Pharm. 2015;41:530–9.
Xie X, Puri VM. Uniformity of powder die filling using a feed shoe: a review. Part Sci Technol. 2007;24:411–26.
Mendez R, Muzzio F, Velazquez C. Study of the effects of feed frames on powder blend properties during the filling of tablet press dies. Powder Technol. 2010;200:105–16.
Schneider L, Sinka IC, Cocks A. Characterisation of the flow behaviour of pharmaceutical powders using a model die–shoe filling system. Powder Technol. 2007;173:59–71.
Sun CC. Quantifying effects of moisture content on flow properties of microcrystalline cellulose using a ring shear tester. Powder Technol. 2016;289:104–8.
Hou H, Sun CC. Quantifying effects of particulate properties on powder flow properties using a ring shear tester. J Pharm Sci. 2008;97:4030–9.
Mills LA, Sinka IC. Effect of particle size and density on the die fill of powders. Eur J Pharm Biopharm. 2013;84:642–52.
Sinka IC, Schneider LCR, Cocks ACF. Measurement of the flow properties of powders with special reference to die fill. Int J Pharm. 2004;280:27–38.
Akseli I, Ladyzhynsky N, Katz J, He X. Development of predictive tools to assess capping tendency of tablet formulations. Powder Technol. 2013;236:139–48.
Sun CC. Setting the bar for powder flow properties in successful high speed tableting. Powder Technol. 2010;201:106–8.
Peeters E, Vanhoorne V, Vervaet C, Remon J-P. Lubricant sensitivity in function of paddle movement in the forced feeder of a high-speed tablet press. Drug Dev Ind Pharm. 2016;42:2078–85.
Lieberman HA. Pharmaceutical dosage forms. 2nd ed. New York: Dekker; 1990.
Schiano S, Chen L, Wu C-Y. The effect of dry granulation on flow behaviour of pharmaceutical powders during die filling. Powder Technol. 2018;337:78–83.
Osei-Yeboah F, Chang S-Y, Sun CC. A critical examination of the phenomenon of bonding area - bonding strength interplay in powder tableting. Pharm Res. 2016;33:1126–32.
Duberg M, Nyström C. Studies on direct compression of tablets XVII. Porosity—pressure curves for the characterization of volume reduction mechanisms in powder compression. Powder Technol. 1986;46:67–75.
Saniocki I, Sakmann A, Leopold CS. Direct compression of ibuprofen-containing powder blends influence of the ibuprofen grade on the flow and compaction properties of an ibuprofen tablet formulation. Pharm Ind. 2012;74:1842–52.
Suresh P, Basu PK. Improving pharmaceutical product development and manufacturing: impact on cost of drug development and cost of goods sold of pharmaceuticals. J Pharm Innov. 2008;3:175–87.
Kawashima Y, Imai M, Takeuchi H, Yamamoto H, Kamiya K, Hino T. Improved flowability and compactibility of spherically agglomerated crystals of ascorbic acid for direct tableting designed by spherical crystallization process. Powder Technol. 2003;130:283–9.
Jivraj M, Martini LG, Thomson CM. An overview of the different excipients useful for the direct compression of tablets. Pharm Sci Technol Today. 2000;3:58–63.
Gohel MC, Jogani PD. A review of co-processed directly compressible excipients. J Pharm Pharm Sci. 2005;8:76–93.
Fartashvand V, Abdullah A, Ali Sadough Vanini S. Effects of high power ultrasonic vibration on the cold compaction of titanium. Ultrason Sonochem. 2017;36:155–61.
Cha HR. Densification of the nanopowder by using ultrasonic vibration compaction. Rev Adv Mater Sci. 2011;28:90–3.
Levina M, Rubinstein MH. The effect of ultrasonic vibration on the compaction characteristics of ibuprofen. Drug Dev Ind Pharm. 2002;28:495–514.
Behrens B-A, Gastan E, Vahed N. Application of tool vibration in die pressing of Ti-powder. Prod Eng Res Devel. 2010;4:545–51.
May RK, Su K, Han L, Zhong S, Elliott JA, Gladden LF, et al. Hardness and density distributions of pharmaceutical tablets measured by terahertz pulsed imaging. J Pharm Sci. 2013;102:2179–86.
Akseli I, Stecula A, He X, Ladyzhynsky N. Quantitative correlation of the effect of process conditions on the capping tendencies of tablet formulations. J Pharm Sci. 2014;103:1652–63.
Mazel V, Busignies V, Diarra H, Tchoreloff P. Lamination of pharmaceutical tablets due to air entrapment: direct visualization and influence of the compact thickness. Int J Pharm. 2015;478:702–4.
Levina M, Rubinstein MH. The effect of ultrasonic vibration on the compaction characteristics of paracetamol. J Pharm Sci. 2000;89:705–23.
Rodriguez L, Cini M, Cavallari C, Passerini N, Saettone M, Fini A, et al. Evaluation of theophylline tablets compacted by means of a novel ultrasound-assisted apparatus. Int J Pharm. 1998;170:201–8.
Kalies A, Özcoban H, Leopold CS. Performance characteristics of a novel vibration technique for the densification of a powder bed within a die of a rotary tablet press - a proof of concept. AAPS PharmSciTech. 2019;20:148.
Schmidt I, Naeve J, Heinrich T. Rotary tablet press and method for pressing tablets in a rotary tablet press; 2011; US9.327.469B2.
Etzler FM, Bramante T, Deanne R, Sienkiewicz S, Chen FJ. Tablet tensile strength: an adhesion science perspective. J Adhes Sci Technol. 2012;25:501–19.
Mooser Schwingungstechnick GmbH. Pneumatic vibrators.; 2019 June 18. Available from: http://www.mooser.net/en/industry-products/pneumatic-vibrators/directional-vibration/piston-vibrators-mkk.html.
Mooser Schwingungstechnick GmbH. Pneumatic vibrators.; 2019 June 18. Available from: https://www.mooser.net/industrieprodukte/druckluftruettler/kreisschwingung/turbinenvibratoren-mtt.html.
Fell JT, Newton JM. Determination of tablet strength by the diametral-compression test. J Pharm Sci. 1970;59:688–91.
Fassihi AR, Kanfer I. Effect of compressibility and powder flow properties on tablet weight variation. Drug Dev Ind Pharm. 2008;12:1947–66.
Sun CC. A novel method for deriving true density of pharmaceutical solids including hydrates and water-containing powders. J Pharm Sci. 2004;93:646–53.
Crouter A, Briens L. The effect of moisture on the flowability of pharmaceutical excipients. AAPS PharmSciTech. 2014;15:65–74.
Sebhatu T, Ahlneck C, Alderborn G. The effect of moisture content on the compression and bond-formation properties of amorphous lactose particles. Int J Pharm. 1997;146:101–14.
Harwood CF. Powder segregation due to vibration. Powder Technol. 1977;16:51–7.
Acknowledgments and Disclosures
The authors would like to thank Lehmann & Voss, Gustav Parmentier, JRS Pharma and Meggle for providing the powder materials.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalies, A., Özcoban, H. & Leopold, C.S. Application of Externally Applied Lower Punch Vibration and its Effects on Tablet Manufacturing. Pharm Res 36, 173 (2019). https://doi.org/10.1007/s11095-019-2711-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2711-7