Abstract
Purpose
In this study we evaluated the utility of in-vitro screening tools for predicting the in-vivo behavior of six cyclic peptides with different solubility and permeability properties (BCS class II and III), intended for oral delivery in presence of permeation enhancer Labrasol.
Methods
An in vitro flux assay was used to assess peptide permeation across a biomimetic, lipid-based membrane and in vivo studies in rats were used to determine oral peptide bioavailability in the presence of Labrasol.
Results
The in vitro flux was significantly increased for BCS class III peptides, while it significantly decreased or remained unchanged for BCS class II peptides with increasing Labrasol concentrations. The different flux responses were attributed to the combination of reduced effective free peptide concentration and increased membrane permeability in the presence of Labrasol. In vivo studies in male Wistar-Hans rats indicated improved oral bioavailability at different extents for all peptides in presence of Labrasol. On comparing the in vitro and in vivo data, a potential direct correlation for BCS class III peptides was seen but not for BCS class II peptides, due to lower free concentrations of peptides in this class.
Conclusion
This study assessed the utility of in vitro screening tools for selecting peptides and permeation excipients early in drug product development.

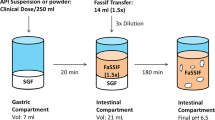
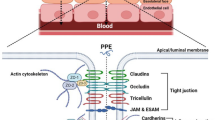
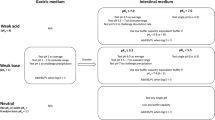
Graphical Abstract Graphical Abstract and Figure 1 contains small text.Graphical Abstract text is made larger. The Figure 1 text cannot be made larger.









Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the Curve
- BCS:
-
Biopharmaceutics Classification System
- COSY:
-
Correlation spectroscopy
- Da:
-
Daltons
- EDTA:
-
Ethylenediaminetetraacetic acid
- HMBC:
-
Heteronuclear Multiple Bond Correlation
- HSQC:
-
Heteronuclear Single Quantum Coherence/Correlation Spectroscopy
- ID:
-
Intra-duodenal
- IV:
-
Intra-venous
- NMR:
-
Nuclear Magnetic Resonance
- NOESY:
-
Nuclear Overhauser Enhancement Spectroscopy
- PAMPA:
-
Parallel Artificial Membrane Permeability Assay
- PBS:
-
Phosphate Buffered Saline
References
David JC, David PF, Spiros L, David P. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81(1):136–47.
Bak A, Leung D, Barrett SE, Forster S, Minnihan EC, Leithead AW, et al. Physicochemical and formulation Developability assessment for therapeutic peptide delivery-a primer. AAPS J. 2015;17(1):144–55.
Bruno BJ, Miller GD, Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Deliv. 2013;4(11):1443–67.
Antosova Z, Mackova M, Kral V, Macek T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27(11):628–35.
Morten Asser K, Bente Juul R, Nozer M, William S, Ehud A, Claus C, et al. Lessons learned from the clinical development of oral peptides. Br J Clin Pharmacol. 2015;79(5):720–32.
Varamini P, Toth I. Recent advances in oral delivery of peptide hormones. Expert Opin Drug Del. 2016;13(4):507–22.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1):3–25.
Andrew TB, Cayla MM, Lokey RS. Form and function in cyclic peptide natural products: a pharmacokinetic perspective. Curr Top Med Chem. 2013;13(7):821–36.
Wang J, Yadav V, Smart AL, Tajiri S, Basit AW. Toward Oral delivery of biopharmaceuticals: an assessment of the gastrointestinal stability of 17 peptide drugs. Mol Pharm. 2015;12(3):966–73.
Nielsen DS, Shepherd NE, Xu W, Lucke AJ, Stoermer MJ, Fairlie DP. Orally absorbed cyclic peptides. Chem Rev. 2017;117(12):8094–128.
Pye CR, Hewitt WM, Schwochert J, Haddad TD, Townsend CE, Etienne L, et al. Nonclassical size dependence of permeation defines bounds for passive adsorption of large drug molecules. J Med Chem. 2017;60(5):1665–72.
David JB, Randall JM. Oral peptide delivery: prioritizing the leading technologies. Ther Deliv. 2011;2(12):1567–73.
Foger F, Kopf A, Loretz B, Albrecht K. A. B-S. correlation of in vitro and in vivo models for the oral absorption of peptide drugs. Amino Acids. 2008;35(1):233–41.
Ahlbach CL, Lexa KW, Bockus AT, Chen V, Crews P, Jacobson MP, et al. Beyond cyclosporine a: conformation-dependent passive membrane permeabilities of cyclic peptide natural products. Future Med Chem. 2015;7(16):2121–30.
Lundquist P, Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv Drug Deliv Rev. 2016;106:256–76.
Borbas E, Balogh A, Bocz K, Muller J, Kiserdei E, Vigh T, et al. In vitro dissolution-permeation evaluation of an electrospun cyclodextrin-based formulation of aripiprazole using muFlux. Int J Pharm. 2015;491(1–2):180–9.
Borbás E, Sinkó B, Tsinman O, Tsinman K, Kiserdei É, Démuth B, et al. Investigation and mathematical description of the real driving force of passive transport of drug molecules from supersaturated solutions. Mol Pharm. 2016;13(11):3816–26.
Jerschow A, Müller N. Suppression of convection artifacts in stimulated-Echo diffusion experiments. Double-stimulated-Echo experiments. J Magn Reson. 1997;125(2):372–5.
H. Wu D, D. Chen A, Johnson C. An Improved Diffusion-Ordered Spectroscopy Experiment Incorporating Bipolar-Gradient Pulses. J Magn Reson Ser A. 1995:260–264.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Karataş A, Yüksel N, Baykara T. Improved solubility and dissolution rate of piroxicam using gelucire 44/14 and labrasol. Farmaco. 2005;60(9):777–82.
Khames A. Investigation of the effect of solubility increase at the main absorption site on bioavailability of BCS class II drug (risperidone) using liquisolid technique. Drug Deliv. 2017;24(1):328–38.
Maher S, Mrsny RJ, Brayden DJ. Intestinal permeation enhancers for oral peptide delivery. Adv Drug Deliv Rev. 2016;106(Pt B:277–319.
Shen Y, Lu Y, Jv M, Hu J, Li Q, Tu J. Enhancing effect of Labrasol on the intestinal absorption of ganciclovir in rats. Drug Dev Ind Pharm. 2011;37(12):1415–21.
Tsinman K, Tsinman O, Lingamaneni R, Zhu S, Riebesehl B, Grandeury A, et al. Ranking Itraconazole formulations based on the flux through artificial lipophilic membrane. Pharm Res. 2018;35(8):161.
Gao Y, Carr RA, Spence JK, Wang WW, Turner TM, Lipari JM, et al. A pH-dilution method for estimation of biorelevant drug solubility along the gastrointestinal tract: application to physiologically based pharmacokinetic modeling. Mol Pharm. 2010;7(5):1516–26.
Fernandez S, Chevrier S, Ritter N, Mahler B, Demarne F, Carrière F, et al. In vitro gastrointestinal lipolysis of four formulations of Piroxicam and Cinnarizine with the self emulsifying excipients Labrasol and Gelucire 44/14. Pharm Res. 2009;26(8):1901–10.
Dahan A, Beig A, Lindley D, Miller JM. The solubility-permeability interplay and oral drug formulation design: two heads are better than one. Adv Drug Deliv Rev. 2016;101:99–107.
Gerhard L, Karen EM, Richard HR. Effect of complex formation on drug absorption III: concentration and drug dependent effect of a nonionic surfactant. J Pharm Sci. 1966;55(4):394–8.
Amidon GE, Higuchi WI, Ho NFH. Theoretical and experimental studies of transport of micelle-solubilized solutes. J Pharm Sci. 1982;71(1):77–84.
Arellano A, Santoyo S, Martn C, Ygartua P. Surfactant effects on the in vitro percutaneous absorption of diclofenac sodium. Eur J Drug Metal Pharmacokinet. 1998;23(2):307–12.
Miller JM, Beig A, Krieg BJ, Carr RA, Borchardt TB, Amidon GE, et al. The solubility-permeability interplay: mechanistic modeling and predictive application of the impact of micellar Solubilization on intestinal permeation. Mol Pharm. 2011;8(5):1848–56.
Rosen MJ, Kunjappu JT. Solubilization by solutions of surfactants:micellar catalysis. In. Surfactants and interfacial phenomena. Wiley: Hoboken; 2012. p. 202–34.
Koga K, Kusawake Y, Ito Y, Sugioka N, Shibata N, Takada K. Enhancing mechanism of Labrasol on intestinal membrane permeability of the hydrophilic drug gentamicin sulfate. Eur J Pharm Biopharm. 2006;64(1):82–91.
Assmus F, Ross A, Fischer H, Seelig J, Seelig A. 31P and 1H NMR studies of the molecular Organization of Lipids in the parallel artificial membrane permeability assay. Mol Pharm. 2017;14(1):284–95.
Paternostre MT, Roux M, Rigaud JL. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 1. Solubilization of large unilamellar liposomes (prepared by reverse-phase evaporation) by triton X-100, octyl glucoside, and sodium cholate. Biochemistry. 1988;27(8):2668–77.
Maria-Angeles U, Felix MG, Alicia A. Structural changes induced by triton X-100 on sonicated phosphatidylcholine liposomes. Eur J Biochem. 1988;173(3):585–8.
Lichtenberg D, Ahyayauch H, Alonso A, Goñi F. Detergent solubilization of lipid bilayers: a balance of driving forces. Trends Biochem Sci. 2013;38(2):85–93.
Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta Biomembr. 2004;1666(1):105–17.
Tsinman K, Tsinman O. Novel HT method for parallel excipient/vehicle formulation studies. In.AAPS. 2013:2013.
Katneni K, Charman SA, Porter CJH. Permeability assessment of poorly water-soluble compounds under solubilizing conditions: the reciprocal permeability approach. J Pharm Sci. 2006;95(10):2170–85.
Buckley ST, Frank KJ, Fricker G, Brandl M. Biopharmaceutical classification of poorly soluble drugs with respect to "enabling formulations". Eur J Pharm Sci. 2013;50(1):8–16.
Sha X, Yan G, Wu Y, Li J, Fang X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur J Pharm Sci. 2005;24(5):477–86.
Chiu Y-Y, Higaki K, Neudeck BL, Barnett JL, Welage LS, Amidon GL. Human Jejunal permeability of Cyclosporin a: influence of surfactants on P-glycoprotein efflux in Caco-2 cells. In. 2003:749–56.
Kino K, Taguchi Y, Yamada K, Komano T, Ueda K. Aureobasidin a, an antifungal cyclic depsipeptide antibiotic, is a substrate for both human MDR1 and MDR2/P-glycoproteins. FEBS Lett. 1996;399(1–2):29–32.
Lin Y, Shen Q, Katsumi H, Okada N, Fujita T, Jiang X, et al. Effects of Labrasol and other pharmaceutical excipients on the intestinal transport and absorption of Rhodamine123, a P-glycoprotein substrate, in rats. Biol Pharm Bull. 2007;30(7):1301–7.
Bravo González RC, Huwyler J, Walter I, Mountfield R, Bittner B. Improved oral bioavailability of cyclosporin a in male Wistar rats: comparison of a Solutol HS 15 containing self-dispersing formulation and a microsuspension. Int J Pharm. 2002;245(1):143–51.
Drewe J, Beglinger C, Kissel T. The absorption site of cyclosporin in the human gastrointestinal tract. Br J Clin Pharmacol. 1992;33(1):39–43.
Fricker G, Drewe J, Vonderscher J, Kissel T, Beglinger C. Enteral absorption of octreotide. Br J Pharmacol. 1992;105(4):783–6.
Busby RW, Kessler MM, Bartolini WP, Bryant AP, Hannig G, Higgins CS, et al. Pharmacologic properties, metabolism, and disposition of Linaclotide, a novel therapeutic peptide approved for the treatment of irritable bowel syndrome with constipation and chronic idiopathic constipation. J Pharmacol Exp Ther. 2013;344(1):196–206.
Acknowledgments and Disclosures
This research was performed as a part of MRL Postdoctoral Research Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A part of this work was presented as poster presentations at the 2017 Gordon Research Conference, Preclinical Form and Formulation for Drug Discovery
Rights and permissions
About this article
Cite this article
Gadgil, P., Alleyne, C., Feng, KI. et al. Assessing the Utility of In Vitro Screening Tools for Predicting Bio-Performance of Oral Peptide Delivery. Pharm Res 36, 151 (2019). https://doi.org/10.1007/s11095-019-2682-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2682-8