Abstract
Purpose
The organic cation transporters (OCTs) and multidrug and toxin extrusions (MATEs) together are regarded as an organic cation transport system critical to the disposition and response of many organic cationic drugs. Patient response to the analgesic morphine, a characterized substrate for human OCT1, is highly variable. This study was aimed to examine whether there is any organic cation transporter-mediated drug and drug interaction (DDI) between morphine and commonly co-administrated drugs.
Methods
The uptake of morphine and its inhibition by six drugs which are commonly co-administered with morphine in the clinic were assessed in human embryonic kidney 293 (HEK293) cells stably expressing OCT1, OCT2 and MATE1. The in vivo interaction between morphine and the select irinotecan was determined by comparing the disposition of morphine in the absence versus presence of irinotecan treatment in mice.
Results
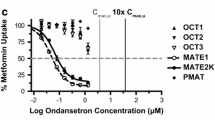
The uptake of morphine in the stable HEK293 cells expressing human OCT1 and OCT2 was significantly increased by 3.56 and 3.04 fold, respectively, than that in the control cells, with no significant uptake increase in the cells expressing human MATE1. All of the six drugs examined, including amitriptyline, fluoxetine, imipramine, irinotecan, ondansetron, and verapamil, were inhibitors of OCT1/2-mediated morphine uptake. The select irinotecan significantly increased the plasma concentrations and decreased hepatic and renal accumulation of morphine in mice.
Conclusions
Morphine is a substrate of OCT1 and OCT2. Clinician should be aware that the disposition of and thus the response to morphine may be altered by co-administration of an OCT1/2 inhibitor, such as irinotecan.




Similar content being viewed by others
References
Andersen G, Christrup L, Sjogren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manag. 2003;25:74–91.
Cherny N, Ripamonti C, Pereira J, Davis C, Fallon M, McQuay H, et al. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 2001;19:2542–54.
Sverrisdottir E, Lund TM, Olesen AE, Drewes AM, Christrup LL, Kreilgaard M. A review of morphine and morphine-6-glucuronide's pharmacokinetic-pharmacodynamic relationships in experimental and clinical pain. Eur J Pharm Sci. 2015;74:45–62.
Wiffen PJ, Wee B, Moore RA. Oral morphine for cancer pain. Cochrane Database Syst Rev:CD003868. 2013.
Babul N, Provencher L, Laberge F, Harsanyi Z, Moulin D. Comparative efficacy and safety of controlled-release morphine suppositories and tablets in cancer pain. J Clin Pharmacol. 1998;38:74–81.
Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686–91.
Morphine in cancer pain: modes of administration. Expert Working Group of the European Association for Palliative Care. BMJ. 1996. 312:823–826.
Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer. 1987;59:850–6.
Brokjaer A, Kreilgaard M, Olesen AE, Simonsson US, Christrup LL, Dahan A, et al. Population pharmacokinetics of morphine and morphine-6-glucuronide following rectal administration--a dose escalation study. Eur J Pharm Sci. 2015;68:78–86.
Coffman BL, Rios GR, King CD, Tephly TR. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab Dispos. 1997;25:1–4.
Shu Y, Leabman MK, Feng B, Mangravite LM, Huang CC, Stryke D, et al. Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc Natl Acad Sci U S A. 2003;100:5902–7.
Tzvetkov MV, dos Santos Pereira JN, Meineke I, Saadatmand AR, Stingl JC, Brockmoller J. Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration. Biochem Pharmacol. 2013;86:666–78.
Fukuda T, Chidambaran V, Mizuno T, Venkatasubramanian R, Ngamprasertwong P, Olbrecht V, et al. OCT1 genetic variants influence the pharmacokinetics of morphine in children. Pharmacogenomics. 2013;14:1141–51.
Balyan R, Zhang X, Chidambaran V, Martin LJ, Mizuno T, Fukuda T, et al. OCT1 genetic variants are associated with postoperative morphine-related adverse effects in children. Pharmacogenomics. 2017;18:621–9.
Koepsell H. Organic cation transporters in intestine, kidney, liver, and brain. Annu Rev Physiol. 1998;60:243–66.
Li Q, Ye Z, Zhu P, Guo D, Yang H, Huang J, et al. Indinavir alters the pharmacokinetics of lamivudine partially via inhibition of multidrug and toxin extrusion protein 1 (MATE1). Pharm Res. 2018;35:14.
Koepsell H. Role of organic cation transporters in drug-drug interaction. Expert Opin Drug Metab Toxicol. 2015;11:1619–33.
Konig J, Muller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65:944–66.
Maeda K. Organic anion transporting polypeptide (OATP)1B1 and OATP1B3 as important regulators of the pharmacokinetics of substrate drugs. Biol Pharm Bull. 2015;38:155–68.
Nies AT, Damme K, Kruck S, Schaeffeler E, Schwab M. Structure and function of multidrug and toxin extrusion proteins (MATEs) and their relevance to drug therapy and personalized medicine. Arch Toxicol. 2016;90:1555–84.
Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang SM, et al. Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs). Toxicol Appl Pharmacol. 2013;273:100–9.
Guo D, Yang H, Li Q, Bae HJ, Obianom O, Zeng S, et al. Selective inhibition on organic cation transporters by carvedilol protects mice from cisplatin-induced nephrotoxicity. Pharm Res. 2018;35:204.
Moraes MO, Lerner FE, Corso G, Bezerra FA, Moraes ME, De Nucci G. Fluoxetine bioequivalence study: quantification of fluoxetine and norfluoxetine by liquid chromatography coupled to mass spectrometry. J Clin Pharmacol. 1999;39:1053–61.
Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, et al. Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013;56:781–95.
Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011:105–67.
Campa D, Gioia A, Tomei A, Poli P, Barale R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther. 2008;83:559–66.
Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of P-glycoprotein in the intestinal absorption and clinical effects of morphine. Clin Pharmacol Ther. 2003;74:543–54.
Tzvetkov MV, Vormfelde SV, Balen D, Meineke I, Schmidt T, Sehrt D, et al. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin Pharmacol Ther. 2009;86:299–306.
Venkatasubramanian R, Fukuda T, Niu J, Mizuno T, Chidambaran V, Vinks AA, et al. ABCC3 and OCT1 genotypes influence pharmacokinetics of morphine in children. Pharmacogenomics. 2014;15:1297–309.
Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422–31.
Tzvetkov MV. OCT1 pharmacogenetics in pain management: is a clinical application within reach? Pharmacogenomics. 2017;18:1515–23.
Sadhasivam S, Krekels EH, Chidambaran V, Esslinger HR, Ngamprasertwong P, Zhang K, et al. Morphine clearance in children: does race or genetics matter? J Opioid Manag. 2012;8:217–26.
Fenner KS, Troutman MD, Kempshall S, Cook JA, Ware JA, Smith DA, et al. Drug-drug interactions mediated through P-glycoprotein: clinical relevance and in vitro-in vivo correlation using digoxin as a probe drug. Clin Pharmacol Ther. 2009;85:173–81.
Song IS, Shin HJ, Shim EJ, Jung IS, Kim WY, Shon JH, et al. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther. 2008;84:559–62.
Chen Y, Li S, Brown C, Cheatham S, Castro RA, Leabman MK, et al. Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet Genomics. 2009;19:497–504.
Wang ZJ, Yin OQ, Tomlinson B, Chow MS. OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics. 2008;18:637–45.
Lazar A, Zimmermann T, Koch W, Grundemann D, Schomig A, Kastrati A, et al. Lower prevalence of the OCT2 Ser270 allele in patients with essential hypertension. Clin Exp Hypertens. 2006;28:645–53.
Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402.
Ivanyuk A, Livio F, Biollaz J, Buclin T. Renal drug transporters and drug interactions. Clin Pharmacokinet. 2017;56:825–92.
Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–51.
Denlinger CS, Blanchard R, Xu L, Bernaards C, Litwin S, Spittle C, et al. Pharmacokinetic analysis of irinotecan plus bevacizumab in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;65:97–105.
Christrup LL. Morphine metabolites. Acta Anaesthesiol Scand. 1997;41:116–22.
Lam AM. Potentially lethal interaction of cimetidine and morphine. Can Med Assoc J. 1981;125:820.
Fujita K, Kubota Y, Ishida H, Sasaki Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J Gastroenterol. 2015;21:12234–48.
Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–87.
Mulgaonkar A, Venitz J, Grundemann D, Sweet DH. Human organic cation transporters 1 (SLC22A1), 2 (SLC22A2), and 3 (SLC22A3) as disposition pathways for fluoroquinolone antimicrobials. Antimicrob Agents Chemother. 2013;57:2705–11.
Acknowledgments and Disclosures
The present study was partially supported by the National Natural Science Foundation (NNSF) of China (81,570,533, Q.L.) and the Natural Science Foundation for Young Scientist of Hunan Province, China (2018JJ3829, Q.L.). Dr. Yan Shu received funding supports from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under Award R01GM099742 (Y.S.) and the US Food and Drug Administration (FDA) under Award U01FD004320 (J.E.P, Y.S.). Dr. Dong Guo is an M-CERSI Scholar (FDA 1U01FD005946). Dr. Yan Shu is a co-founder for and owns equity in Optivia Biotechnology. Designed Research: Yan Shu, Qing Li, Wei Zhang, James E. polli, Honghao Zhou. Performed Research: Peng Zhu, Zhi Ye, Zongping Xiong, Shiqiong Huang, Dong Guo. Analyzed Data: Peng Zhu, Zhi Ye, Qing Li, Dong Guo, Jun Guo. Wrote Manuscript: Peng Zhu, Qing Li, Yan Shu. All authors approved the version to be submitted.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhu, P., Ye, Z., Guo, D. et al. Irinotecan Alters the Disposition of Morphine Via Inhibition of Organic Cation Transporter 1 (OCT1) and 2 (OCT2). Pharm Res 35, 243 (2018). https://doi.org/10.1007/s11095-018-2526-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2526-y