Abstract
Purpose
To investigate influence of inflammation on metabolism and pharmacokinetics (PK) of midazolam (MDZ) and construct a semi-physiologically based pharmacokinetic (PBPK) model to predict PK in mice with inflammatory disease.
Methods
Glucose-6-phosphate isomerase (GPI)-mediated inflammation was used as a preclinical model of arthritis in DBA/1 mice. CYP3A substrate MDZ was selected to study changes in metabolism and PK during the inflammation. The semi-PBPK model was constructed using mouse physiological parameters, liver microsome metabolism, and healthy animal PK data. In addition, serum cytokine, and liver-CYP (cytochrome P450 enzymes) mRNA levels were examined.
Results
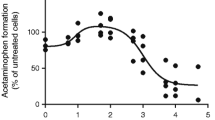
The in vitro metabolite formation rate was suppressed in liver microsomes prepared from the GPI-treated mice as compared to the healthy mice. Further, clearance of MDZ was reduced during inflammation as compared to the healthy group. Finally, the semi-PBPK model was used to predict PK of MDZ after GPI-mediated inflammation. IL-6 and TNF-α levels were elevated and liver-cyp3a11 mRNA was reduced after GPI treatment.
Conclusion
The semi-PBPK model successfully predicted PK parameters of MDZ in the disease state. The model may be applied to predict PK of other drugs under disease conditions using healthy animal PK and liver microsomal data as inputs.






Similar content being viewed by others
Change history
25 July 2018
One of the authors has his name incorrectly indexed in PubMed and SpringerLink as “Laird Forrest M” (last name “Laird Forrest”). His name should index as “Forrest M. Laird” with last name as “Forrest”.
Abbreviations
- CAR:
-
Constitutive androstane receptor
- CYP:
-
Cytochrome P450
- DBS:
-
Dried blood spot
- GPI:
-
Glucose-6-phosphate isomerase
- HNF-4α:
-
Hepatic nuclear factor-4α
- HLM:
-
Human liver microsomes
- IV:
-
Intravenous
- LC-MS:
-
Liquid Chromatography-Mass Spectrometry
- MDZ:
-
Midazolam
- MLM:
-
Mouse liver microsomes
- NCA:
-
Non-compartmental analysis
- NCE:
-
New chemical entity
- PBPK:
-
Physiologically based pharmacokinetic
- PK:
-
Pharmacokinetics
- PO:
-
Oral
- PXR:
-
Pregnane X receptor
- qPCR:
-
Quantitative polymerase chain reaction
- SCID:
-
Severe combined immune deficient
References
Morgan E. Impact of infectious and inflammatory disease on cytochrome P450–mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther. 2009;85(4):434–8.
Frye RF, Schneider VM, Frye CS, Feldman AM. Plasma levels of TNF-α and IL-6 are inversely related to cytochrome P450-dependent drug metabolism in patients with congestive heart failure. J Card Fail. 2002;8(5):315–9.
Coutant D, Kulanthaivel P, Turner P, Bell R, Baldwin J, Wijayawardana S, et al. Understanding disease–drug interactions in Cancer patients: implications for dosing within the therapeutic window. Clin Pharmacol Ther. 2015;98(1):76–86.
Robertson G, Liddle C, Clarke S. Inflammation and altered drug clearance in Cancer: transcriptional repression of a human CYP3A4 transgene in tumor-bearing mice. Clin Pharmacol Ther. 2008;83(6):894–7.
Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Disposition. 2007;35(9):1687–93.
Xu Y, Hijazi Y, Wolf A, Wu B, Sun YN, Zhu M. Physiologically based pharmacokinetic model to assess the influence of Blinatumomab-mediated cytokine elevations on cytochrome P450 enzyme activity. CPT: pharmacometrics & systems pharmacology. 2015;4(9):507–15.
Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2(6):875–94.
Matsumoto I, Zhang H, Yasukochi T, Iwanami K, Tanaka Y, Inoue A, et al. Therapeutic effects of antibodies to tumor necrosis factor-alpha, interleukin-6 and cytotoxic T-lymphocyte antigen 4 immunoglobulin in mice with glucose-6-phosphate isomerase induced arthritis. Arthritis Res Ther. 2008;10(3):R66.
Palmqvist N, Siller M, Klint C, Sjödin A. A human and animal model-based approach to investigating the anti-inflammatory profile and potential of the 5-HT 2B receptor antagonist AM1030. J Inflamm. 2016;13(1):20.
Gandhi A, Guo T, Shah P, Moorthy B, Chow DL, Hu M, et al. CYP3A-dependent drug metabolism is reduced in bacterial inflammation in mice. Br J Pharmacol. 2012;166(7):2176–87.
Kato R, Yamashita S, Moriguchi J, Nakagawa M, Tsukura Y, Uchida K, et al. Changes of midazolam pharmacokinetics in Wistar rats treated with lipopolysaccharide: relationship between total CYP and CYP3A2. Innate Immun. 2008;14(5):291–7.
Kajikawa N, Doi M, Kusaba J-i, Aiba T. Effect of carrageenan-induced acute peripheral inflammation on the pharmacokinetics and hepatic metabolism of midazolam in rats. Drug Metab Pharmacokinet. 2014;29(5):400–6.
Perloff MD, von Moltke LL, Cotreau MM, Greenblatt DJ. Unchanged cytochrome P450 3A (CYP3A) expression and metabolism of midazolam, triazolam, and dexamethasone in mdr (−/−) mouse liver microsomes. Biochem Pharmacol. 1999;57(11):1227–32.
Samuelsson K, Pickup K, Sarda S, Swales JG, Morikawa Y, Schulz-Utermoehl T, et al. Pharmacokinetics and metabolism of midazolam in chimeric mice with humanised livers. Xenobiotica. 2012;42(11):1128–37.
Patki KC, von Moltke LL, Greenblatt DJ. In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes p450: role of cyp3a4 and cyp3a5. Drug Metab Disposition. 2003;31(7):938–44.
Bockermann R, Schubert D, Kamradt T, Holmdahl R. Induction of a B-cell-dependent chronic arthritis with glucose-6-phosphate isomerase. Arthrit Res Ther. 2005;7(6):R1316–R24.
Kamath S, Kummerow F, Narayan KA. A simple procedure for the isolation of rat liver microsomes. FEBS Lett. 1971;17(1):90–2.
Elovaara E, Mikkola J, Luukkanen L, Antonio L, Fournel-Gigleux S, Burchell B, et al. Assessment of catechol induction and glucuronidation in rat liver microsomes. Drug Metab Disposition. 2004;32(12):1426–33.
Smith PK, Krohn RI, Hermanson G, Mallia A, Gartner F, Provenzano M, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85.
Granvil CP, Yu A-M, Elizondo G, Akiyama TE, Cheung C, Feigenbaum L, et al. Expression of the human CYP3A4 gene in the small intestine of transgenic mice: in vitro metabolism and pharmacokinetics of midazolam. Drug Metab Disposition. 2003;31(5):548–58.
Kuze J, Mutoh T, Takenaka T, Morisaki K, Nakura H, Hanioka N, et al. Separate evaluation of intestinal and hepatic metabolism of three benzodiazepines in rats with cannulated portal and jugular veins: comparison with the profile in non-cannulated mice. Xenobiotica. 2009;39(11):871–80.
Zhang W, Han F, Guo P, Zhao H, Lin ZJ, Huang M-Q, et al. Simultaneous determination of tolbutamide, omeprazole, midazolam and dextromethorphan in human plasma by LC–MS/MS—A high throughput approach to evaluate drug–drug interactions. J Chromatogr B. 2010;878(15):1169–77.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84.
Kirman C, Hays S, Aylward L, Suh M, Harris M, Thompson C, et al. Physiologically based pharmacokinetic model for rats and mice orally exposed to chromium. Chem Biol Interact. 2012;200(1):45–64.
Masyuk TV, Ritman EL, LaRusso NF. Hepatic artery and portal vein remodeling in rat liver: vascular response to selective cholangiocyte proliferation. Am J Pathol. 2003;162(4):1175–82.
Kuze J, Mutoh T, Takenaka T, Oda N, Hanioka N, Narimatsu S. Evaluation of animal models for intestinal first-pass metabolism of drug candidates to be metabolized by CYP3A enzymes via in vivo and in vitro oxidation of midazolam and triazolam. Xenobiotica. 2013;43(7):598–606.
Barter ZE, Bayliss MK, Beaune PH, Boobis AR, Carlile DJ, Edwards RJ, et al. Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human micro-somal protein and hepatocellularity per gram of liver. Curr Drug Metab. 2007;8(1):33–45.
Cubitt HE, Houston JB, Galetin A. Prediction of human drug clearance by multiple metabolic pathways: integration of hepatic and intestinal microsomal and cytosolic data. Drug Metab Disposition. 2011;39(5):864–73.
Zhang X, Quinney SK, Gorski JC, Jones DR, Hall SD. Semiphysiologically based pharmacokinetic models for the inhibition of midazolam clearance by diltiazem and its major metabolite. Drug Metab Disposition. 2009;37(8):1587–97.
Yang J, Jamei M, Yeo KR, Tucker GT, Rostami-Hodjegan A. Prediction of intestinal first-pass drug metabolism. Curr Drug Metab. 2007;8(7):676–84.
Cleton A, Mazee D, Voskuyl R, Danhof M. Rate of change of blood concentrations is a major determinant of the pharmacodynamics of midazolam in rats. Br J Pharmacol. 1999;127(1):227–35.
Wang J, Xia S, Xue W, Wang D, Sai Y, Liu L, et al. A semi-physiologically-based pharmacokinetic model characterizing mechanism-based auto-inhibition to predict stereoselective pharmacokinetics of verapamil and its metabolite norverapamil in human. Eur J Pharm Sci. 2013;50(3):290–302.
Chowdhury F, Williams A, Johnson P. Validation and comparison of two multiplex technologies, Luminex® and mesoscale discovery, for human cytokine profiling. J Immunol Methods. 2009;340(1):55–64.
Lu J, Goldsmith M-R, Grulke CM, Chang DT, Brooks RD, Leonard JA, et al. Developing a physiologically-based pharmacokinetic model knowledgebase in support of provisional model construction. PLoS Comput Biol. 2016;12(2):e1004495.
Czerwiński M, Kazmi F, Parkinson A, Buckley DB. Anti-CD28 monoclonal antibody-stimulated cytokines released from blood suppress CYP1A2, CYP2B6 and CYP3A4 in human hepatocytes in vitro. Drug Metab Disposition. 2015;43(1):42–52.
Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Disposition. 2011;39(8):1415–22.
Machavaram K, Almond L, Rostami-Hodjegan A, Gardner I, Jamei M, Tay S, et al. A physiologically based pharmacokinetic modeling approach to predict disease–drug interactions: suppression of CYP3A by IL-6. Clin Pharmacol Ther. 2013;94(2):260–8.
Charles P, Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C, et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-α therapy in rheumatoid arthritis. J Immunol. 1999;163(3):1521–8.
Lee J-I, Zhang L, Men AY, Kenna LA, Huang S-M. CYP-mediated therapeutic protein-drug interactions. Clin Pharmacokinet. 2010;49(5):295–310.
De Vries J, Rudi J, Walter-Sack I, Conradi R. The determination of total and unbound midazolam in human plasma. A comparison of high performance liquid chromatography, gas chromatography and gas chromatography/mass spectrometry. Biomed Chromatogr. 1990;4(1):28–33.
Reed MD, Myers CM, Blumer JL. Influence of midazolam on the protein binding of ketorolac. Curr Ther Res. 2001;62(8):558–65.
Higai K, Azuma Y, Aoki Y, Matsumoto K. Altered glycosylation of α 1-acid glycoprotein in patients with inflammation and diabetes mellitus. Clin Chim Acta. 2003;329(1):117–25.
Piafsky KM, Borgå O, Odar-Cederlöf I, Johansson C, Sjöqvist F. Increased plasma protein binding of propranolol and chlorpromazine mediated by disease-induced elevations of plasma α1 acid glycoprotein. N Engl J Med. 1978;299(26):1435–9.
Heizmann P, Ziegler W. Excretion and metabolism of 14C-midazolam in humans following oral dosing. Arzneimittelforschung. 1981;31(12a):2220–3.
Woo GK, Williams T, Kolis S, Warinsky D, Sasso G, Schwartz M. Biotransformation of [14C] midazolam in the rat in vitro and in vivo. Xenobiotica. 1981;11(6):373–84.
Thummel KE, O'shea D, Paine MF, Shen DD, Kunze KL, Perkins JD, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59(5):491–502.
Acknowledgements
The authors acknowledge Michael Mohutsky for help with the in vitro metabolism work, Tom Kern (Covance Inc.) for conducting in vivo pharmacokinetic experiments, George Searfoss for CYP mRNA measurements, Bridget Morse for suggestions regarding the semi-PBPK model, and Daniel Mudra for critically reading the manuscript and providing suggestions. Eli Lilly provided support for an internship by NV and funded laboratory and animal studies. NV and MLF were partially supported by a grant from NIH (R01CA173292, PI: Forrest) during analysis and development of the model. NV was partially supported by a Higuchi Fellowship and the Department of Pharmaceutical Chemistry, The University of Kansas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None to declare.
Electronic supplementary material
ESM 1
(DOCX 2719 kb)
Rights and permissions
About this article
Cite this article
Varkhede, N., Patel, N., Chang, W. et al. A Semi-Physiologically Based Pharmacokinetic Model Describing the Altered Metabolism of Midazolam Due to Inflammation in Mice. Pharm Res 35, 162 (2018). https://doi.org/10.1007/s11095-018-2447-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2447-9