Abstract
Purpose
Planned reproduction in cattle involves regulation of estrous cycle and the use of artificial insemination. Cycle control includes the administration of exogenous progesterone during 5–8 days in a controlled manner allowing females to synchronize their ovulation. Several progesterone delivery systems are commercially available but they have several drawbacks. The aim of the present contribution was to evaluate chitosan microparticles entrapping progesterone as an alternative system.
Methods
Microparticles were prepared by spray drying. The effect of formulation parameters and experimental conditions on particle features and delivery was studied. A mathematical model to predict progesterone plasma concentration in animals was developed and validated with experimental data.
Results
Microparticle size was not affected by formulation parameters but sphericity enhances as Tween 80 content increases and it impairs as TPP content rises. Z potential decreases as phosphate content rises. Particles remain stable in acidic solution but the addition of surfactant is required to stabilize dispersions in neutral medium. Encapsulation efficiencies was 69–75%. In vitro delivery studies showed burst and diffusion-controlled phases, being progesterone released faster at low pH. In addition, delivery extend in cows was affected mainly by particle size and hormone initial content, while the amount injected altered plasma concentration. Theoretical predictions with excellent accuracy were obtained.
Conclusion
The mathematical model developed can help to find proper particle features to reach specific delivery rates in the animals. This not only save time, money and effort but also minimized experimentation with animals which is desired from an ethical point of view.







Similar content being viewed by others
Abbreviations
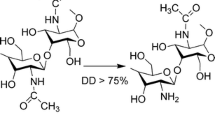
- DD:
-
Deacetylation degree (DD)
- DSC:
-
Differential Scanning Calorimetry
- EEf:
-
Encapsulation efficiency
- FT-IR:
-
Fourier Transform Infrared Spectroscopy
- HPLC:
-
High Performance Liquid Chromatography
- SEM:
-
Scanning Electron Microscopy
- TPP:
-
Tripolyphosphate
References
Helbling IM, Luna JA. Progesterone administration in planned reproduction of cattle. In: Rivera C, editor. Progesterone: functions, uses and research insights. USA: Nova Science Publishers, Inc.; 2017. p. 69–125.
Helbling IM, Luna JA. Progesterone administration in planned reproduction of cattle. Int J Med Biol Front. 2017;23:31–71.
Rathbone MJ, Kinder JE, Fike K, Kojima F, Clopton D, Ogle CR, et al. Recent advances in bovine reproductive endocrinology and physiology and their impact on drug delivery system design for the control of the estrous cycle in cattle. Adv Drug Deliv Rev. 2001;50(3):277–320.
Roelofs J, Lopez-Gatius F, Hunter RH, van Eerdenburg FJ, Hanzen C. When is a cow in estrus? Clinical and practical aspects. Theriogenology. 2010;74(3):327–44.
Lucy MC, McDougall S, Nation DP. The use of hormonal treatments to improve the reproductive performance of lactating dairy cows in feedlot or pasture-based management systems. Anim Reprod Sci. 2004;82–83:495–512.
Weibel MI, Badano JM, Rintoul I. Technological evolution of hormone delivery systems for estrous synchronization in cattle. Int J Livest Res. 2014;4(1):20–40.
Bridges GA, Lake SL. Comparison of the CIDR Select and 5-day Select Synch + CIDR protocols that included limited estrus detection and timed insemination for synchronizing estrus in beef heifers. Prof Anim Sci. 2011;27(2):141–6.
Muth-Spurlock AM, Poole DH, Whisnant CS. Comparison of pregnancy rates in beef cattle after a fixed-time AI with once- or twice-used controlled internal drug release devices. Theriogenology. 2016;85(3):447–51.
Gunn D, Ahmadzadeh A, Glaze B. Effect of duration of progesterone-releasing device (controlled internal drug-release insert) treatment on reproductive performance in beef cows. Prof Anim Sci. 2011;27(2):147–51.
van Werven T, Waldeck F, Souza AH, Floch S, Englebienne M. Comparison of two intravaginal progesterone releasing devices (PRID-Delta vs CIDR) in dairy cows: blood progesterone profile and field fertility. Anim Reprod Sci. 2013;138(3–4):143–9.
Colazo MG, Mapletoft RJ. A review of current timed-AI (TAI) programs for beef and dairy cattle. Can Vet J. 2014;55(8):772–80.
Helbling IM, Ibarra JCD, Luna JA. Mathematical modeling of drug delivery from silicone devices used in bovine estrus synchronization. In: Tiwari A, Soucek MD, editors, Concise encyclopedia of high performance silicones. USA: John Wiley & Sons, Inc.; 2014. p. 225–42.
Leimann FV, Biz MH, Kaufmann KC, Maia WJ, Honçalves OH, Cardozo Filho L, et al. Characterization of progesterone loaded biodegradable blend polymeric nanoparticles. Cienc Rural. 2015;45:2082–8.
Almomen A, Cho S, Yang CH, Li Z, Jarboe EA, Peterson CM, et al. Thermosensitive progesterone hydrogel: a safe and effective new formulation for vaginal application. Pharm Res. 2015;32(7):2266–79.
Turino LN, Mariano RN, Boimvaser S, Luna JA. In situ-formed microparticles of PLGA from O/W emulsions stabilized with PVA: encapsulation and controlled release of progesterone. J Pharm Innov. 2014;9(2):132–40.
Prasad SGB, Gupta VRM, Devanna N, Jayasurya K. Microspheres as drug delivery system – a review. JGTPS. 2014;5(3):1961–72.
Faisant N, Akiki J, Siepmann F, Benoit JP, Siepmann J. Effects of the type of release medium on drug release from PLGA-based microparticles: experiment and theory. Int J Pharm. 2006;314(2):189–97.
Latha MS, Lal AV, Kumary TV, Sreekumar R, Jayakrishnan A. Progesterone release from glutaraldehyde cross-linked casein microspheres: in vitro studies and in vivo response in rabbits. Contraception. 2000;61(5):329–34.
Yang Q, Owusu-Ababio G. Biodegradable progesterone microsphere delivery system for osteoporosis therapy. Drug Dev Ind Pharm. 2000;26(1):61–70.
Wu XS. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers: part III. Drug delivery application. Artif Cells Blood Substit Immobil Biotechnol. 2004;32(4):575–91.
Rosilio V, Benoit JP, Deyme M, Thies C, Madelmont G. A physicochemical study of the morphology of progesterone-loaded microspheres fabricated from poly(D,L-lactide-co-glycolide). J Biomed Mater Res. 1991;25(5):667–82.
Hill VL, Passerini N, Craig DQM, Vickers M, Anwar J, Feely LC. Investigation of progesterone loaded Poly(D,L-Lactide) microspheres using TMDSC, SEM and PXRD. J Therm Anal Calorim. 1998;54(2):673–85.
Chiappetta D, Legaspi MJ, Niselman V, Pasquali R, Gergic E, Rodríguez Llimós AC, et al. Microesferas biodegradables de poli(D,L-láctico) conteniendo progesterona (Biodegradable microspheres of poly(D,L-lactide) containing progesterone). Ars Pharm. 2005;46(4):383–98.
Aso Y, Yoshioka S, Terao T. Effects of storage on the physicochemical properties and release characteristics of progesterone-loaded poly(l-lactide) microspheres. Int J Pharm. 1993;93(1):153–9.
Orienti I, Zecchi V. Progesterone-loaded albumin microparticles. J Control Release. 1993;27(1):1–7.
Courteille F, Benoit JP, Thies C. The morphology of progesterone-loaded polystyrene microspheres. J Control Release. 1994;30(1):17–26.
Al-Obaidi H, Ke P, Brocchini S, Buckton G. Characterization and stability of ternary solid dispersions with PVP and PHPMA. Int J Pharm. 2011;419(1–2):20–7.
Gangrade N, Price JC. Poly(hydroxybutyrate-hydroxyvalerate) microspheres containing progesterone: preparation, morphology and release properties. J Microencapsul. 1991;8(2):185–202.
Jameela SR, Kumary TV, Lal AV, Jayakrishnan A. Progesterone-loaded chitosan microspheres: a long acting biodegradable controlled delivery system. J Control Release. 1998;52(1–2):17–24.
Patel KS, Patel MB. Preparation and evaluation of chitosan microspheres containing nicorandil. Int J Pharm Investig. 2014;4(1):32–7.
Islam MA, Firdous J, Choi YJ, Yun CH, Cho CS. Design and application of chitosan microspheres as oral and nasal vaccine carriers: an updated review. Int J Nanomedicine. 2012;7:6077–93.
Barakat NS, Almurshedi AS. Preparation and characterization of chitosan microparticles for oral sustained delivery of gliclazide: in vitro/in vivo evaluation. Drug Dev Res. 2011;72(3):235–46.
Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm. 2002;249(1–2):165–74.
Mitra A, Dey B. Chitosan microspheres in novel drug delivery systems. Indian J Pharm Sci. 2011;73(4):355–66.
Mengatto LN, Helbling IM, Luna JA. Recent advances in chitosan films for controlled release of drugs. Recent Pat Drug Deliv Formul. 2012;6(2):156–70.
Niknejad H, Mahmoudzadeh R. Comparison of different crosslinking methods for preparation of docetaxel-loaded albumin nanoparticles. Iran J Pharm Res. 2015;14(2):385–94.
Kim M, Takaoka A, Hoang QV, Trokel SL, Paik DC. Pharmacologic alternatives to riboflavin photochemical corneal cross-linking: a comparison study of cell toxicity thresholds. Invest Ophthalmol Vis Sci. 2014;55(5):3247–57.
Stulzer HK, Tagliari MP, Parize AL, Silva MAS, Laranjeira MCM. Evaluation of cross-linked chitosan microparticles containing acyclovir obtained by spray-drying. Mater Sci Eng C. 2009;29(2):387–92.
Shu XZ, Zhu KJ. Chitosan/gelatin microspheres prepared by modified emulsification and ionotropic gelation. J Microencapsul. 2001;18(2):237–45.
Liu W, Wu WD, Selomulya C, Chen XD. Uniform chitosan microparticles prepared by a novel spray-drying technique. Int J Chem Eng. 2011;2011:7.
Esposito E, Roncarati R, Cortesi R, Cervellati F, Nastruzzi C. Production of Eudragit microparticles by spray-drying technique: influence of experimental parameters on morphological and dimensional characteristics. Pharm Dev Technol. 2000;5(2):267–78.
de Alvarenga ES, Pereira de Oliveira C, Roberto Bellato C. An approach to understanding the deacetylation degree of chitosan. Carbohydr Polym. 2010;80(4):1155–60.
Barreiros FM, Ferreira PJ, Figueiredo MM. Calculating shape factors from particle sizing data. Part Part Syst Charact. 1996;13(6):368–73.
Moura MJ, Martins SP, Duarte BPM. Production of chitosan microparticles cross-linked with genipin – Identification of factors influencing size and shape properties. Biochem Eng J. 2015;104:82–90.
Helbling IM, Ibarra JCD, Luna JA. Evaluation and optimization of progesterone release from intravaginal rings using response surface methodology. J Drug Delivery Sci Technol. 2015;29:218–25.
Helbling IM, Ibarra JC, Luna JA. The optimization of an intravaginal ring releasing progesterone using a mathematical model. Pharm Res. 2014;31(3):795–808.
Mariano RN, Turino LN, Cabrera MI, Scándolo DE, Maciel MG, Grau RJA. A simple pharmacokinetic model linking plasma progesterone concentrations with the hormone released from bovine intravaginal inserts. Res Vet Sci. 2010;89(2):250–6.
Sakar F, Ozer AY, Erdogan S, Ekizoglu M, Kart D, Ozalp M, et al. Nano drug delivery systems and gamma radiation sterilization. Pharm Dev Technol. 2017;22(6):775–84.
Desai KG, Park HJ. Study of gamma-irradiation effects on chitosan microparticles. Drug Deliv. 2006;13(1):39–50.
Helbling IM, Ibarra JC, Luna JA. The use of cellulose membrane to eliminate burst release from intravaginal rings. AAPS J. 2016;18(4):960–71.
Mu L, Feng SS. Fabrication, characterization and in vitro release of paclitaxel (Taxol) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J Control Release. 2001;76(3):239–54.
Duclos R, Saiter JM, Grenet J, Orecchioni AM. Polymorphism of progesterone. J Therm Anal Calorim. 1991;37(8):1869–75.
Muramatsu M, Iwahashi M, Takeuchi U. Thermodynamic relationship between α- and β-forms of crystalline progesterone. J Pharm Sci. 1979;68(2):175–7.
Helbling IM, Ibarra JC, Luna JA. Application of the refined integral method in the mathematical modeling of drug delivery from one-layer torus-shaped devices. Int J Pharm. 2012;423(2):240–6.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33.
Pillay V, Fassihi R. Evaluation and comparison of dissolution data derived from different modified release dosage forms: an alternative method. J Control Release. 1998;55:45–55.
Moore JW, Flanner HH. Mathematical comparison of dissolution profiles. Pharm Technol. 1996;20:64–74.
Helbling IM, Luna JA, Cabrera MI. Mathematical modeling of drug delivery from torus-shaped single-layer devices. J Control Release. 2011;149(3):258–63.
Turino LN, Luna JA, Grau RJA, Cabrera MI. Desarrollo de sistemas inyectables biodegradables con formación In situ para la liberación sostenida de drogas veterinarias. Santa Fe: Universidad Nacional del Litoral; 2012.
ACKNOWLEDGMENTS AND DISCLOSURES
uthors wish to express their gratitude to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and to Universidad Nacional del Litoral (UNL) of Argentina for the financial support granted to this contribution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Helbling, I.M., Busatto, C.A., Fioramonti, S.A. et al. Preparation of TPP-crosslinked chitosan microparticles by spray drying for the controlled delivery of progesterone intended for estrus synchronization in cattle. Pharm Res 35, 66 (2018). https://doi.org/10.1007/s11095-018-2363-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2363-z