Abstract
Purpose
The study evaluates the use of new in vitro primary human cell-based organotypic small intestinal (SMI) microtissues for predicting intestinal drug absorption and drug-drug interaction.
Methods
The SMI microtissues were reconstructed using human intestinal fibroblasts and enterocytes cultured on a permeable support. To evaluate the suitability of the intestinal microtissues to model drug absorption, the permeability coefficients across the microtissues were determined for a panel of 11 benchmark drugs with known human absorption and Caco-2 permeability data. Drug-drug interactions were examined using efflux transporter substrates and inhibitors.
Results
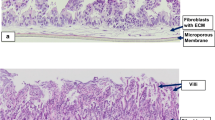
The 3D–intestinal microtissues recapitulate the structural features and physiological barrier properties of the human small intestine. The microtissues also expressed drug transporters and metabolizing enzymes found on the intestinal wall. Functionally, the SMI microtissues were able to discriminate between low and high permeability drugs and correlated better with human absorption data (r2 = 0.91) compared to Caco-2 cells (r2 = 0.71). Finally, the functionality of efflux transporters was confirmed using efflux substrates and inhibitors which resulted in efflux ratios of >2.0 fold and by a decrease in efflux ratios following the addition of inhibitors.
Conclusion
The SMI microtissues appear to be a useful pre-clinical tool for predicting drug bioavailability of orally administered drugs.











Similar content being viewed by others
Abbreviations
- Ω:
-
Ohm
- 2D:
-
Two dimensional
- 3D:
-
Three dimensional
- Ǻ:
-
Angstrom
- (A):
-
Apical
- ABCB1:
-
ATP binding cassette subfamily B member 1
- ABCC1:
-
ATP Binding Cassette Subfamily C Member 1
- ABCC2:
-
ATP Binding Cassette Subfamily C Member 2
- ABCG2:
-
ATP-binding cassette sub-family G member 2
- ADR:
-
Adverse drug reaction
- ALI:
-
Air–liquid interface
- (B):
-
Basolateral
- BCRP:
-
Breast cancer resistance protein
- BCS:
-
Biopharmaceutical Classification System
- CDCF:
-
5(6)-carboxy-2′,7′-dichlorofluorescein (CDCF)
- cDNA:
-
Complementary DNA
- CK:
-
Cytokeratin
- Cq:
-
PCR cycles
- Ct:
-
Threshold cycle
- CYP450:
-
Cytochrome P450
- DDI:
-
Drug-drug interaction
- FDA:
-
Food and Drug Administration
- FT:
-
Full-thickness
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GI:
-
Gastrointestinal
- HPLC:
-
High performance liquid chromatography
- H:
-
Hour
- IIAM:
-
International Institute for the Advancement of Medicine
- LC/MS:
-
Liquid chromatography–mass spectrometry
- LY:
-
Lucifer Yellow
- MDCK:
-
Madin-Darby canine kidney
- MDR1:
-
Multi-drug resistance gene (MDR)-1
- Met:
-
Metabolite
- Min:
-
Minutes
- MRP-1:
-
Multidrug-resistance associated protein-1
- MRP-2:
-
Multidrug-resistance associated protein-2
- MS MRM:
-
Mass spectroscopy multiple reaction monitoring
- MTT:
-
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide
- OPO:
-
Organ Procurement Organization
- Papp :
-
Apparent permeability coefficient
- P-gp:
-
p-Glycoprotein
- PR:
-
Parental
- PT:
-
Partial thickness
- qPCR:
-
Quantitative polymerase chain reaction
- RFU:
-
Relative fluorescence unit
- RNA:
-
Ribonucleic acid
- RT:
-
Room temperature
- RT-PCR:
-
Reverse Transcription Polymerase Chain Reaction
- SEM:
-
Scanning electron microscopy
- SMI:
-
Small intestine
- TEER:
-
Transepithelial electrical resistance
- TEM:
-
Transmission electron microscopy
- TTT:
-
TEER of treated tissues
- TUT:
-
TEER of untreated tissues
References
Pretorius E, Bouic PJD. Permeation of four oral drugs through the human intestinal mucosa. AAPS PharmSciTech. 2009;10:270–5.
Brendon M, Baker BM, Christopher S, Chen CS. Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–24.
Loriot Y, Perlemuter G, Malka D, Penault-Llorca F, Boige V, Deutsch E, et al. Drug insight: gastrointestinal and hepatic adverse effects of molecular-targeted agents in cancer therapy. Nat Clin Pract Oncol. 2008;5:268–78.
Hubatsch I, Ragnarsson EGE, Artursson P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc. 2007;2:2111–9.
Lee MKK, Dilq. Drug development in cell culture: crosstalk from the industrial prospects. J Bioequivalence Bioavailab. 2014;6:O96–O114.
Volpe DA. Drug-permeability and transporter assays in Caco-2 and MDCK cell lines. Future Med Chem. 2011;3:2063–77.
Gupta V, Doshi N, Mitragotri S. Permeation of insulin, calcitonin, and exenatide across Caco-2 monolayers: measurement using rapid 3-day system. PLoS One. 2013;8:e77136.
Tavelin S, Taipalensuu J, Söderberg L, Morrison R, Chong S, Artursson P. Prediction of the oral absorption of low permeability drugs using small intestine-like 2/4/A1 cell monolayers. Pharm Res. 2003;20:397–405.
Ferrec EL, Chesne C, Artusson P, Brayden D, Fabre G, Gires P, et al. In vitro models of the intestinal barrier. The report and recommendations of ECVAM workshop 46. ATLA. 2001;29:649–68.
Huang Y, Adams MC. An in vitro model for investigating intestinal adhesion of potential dairy propionibacteria probiotic strains using cell lineC2BBe1. Lett Appl Microbiol. 2003;36:213e216.
Fang Y, Eglen RM. Three-dimensional cell cultures in drug discovery and development. SLAS Disc. 2017;22:456–72.
Mathur A, Loskill P, Shao K, Huebsch N, Hong SG, Marcus MG, et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep. 2017;5:8883.
Li AP. Preclinical in vitro screening assays for drug-like properties. Drug Discov Today. 2005;2:179–85.
Amdion GL, Lennerlas H, Shah VP, Crison JR. A theoretical basis for biopharmaceutics drug classification: the correlation of in vitro drug product dissolution and in vivo drug bioavailability. Pharm Res. 1995;12:413–20.
http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072101.pdf.
Maschmeyer I, Hasenberg T, Jaenicke A, Lindner M, Lorenz AK, Zech J, et al. Chip-based human liver-intestine and liver-skin co-cultures - a first step toward systemic repeated dose substance testing in vitro. Eur J Pharm Biopharm. 2015;95:77–87.
Wang Z, Hop CE, Leung KH, Pang J. Determination of in vitro permeability of drug candidates through a caco-2 cell monolayer by liquid chromatography/tandem mass spectrometry. J Mass Spectrom. 2000;35:71–6.
Mosmann T. Rapid calorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Ito S, Karnovsky MJ. Formaldehyde-glutaraldehyde fixative containing trinitro compounds. J Cell Biol. 1968;39:168–169-A.
Zimmermann C. Expression of drug transporters in intestine and blood. 2005. Doctoral thesis. http://edoc.unibas.ch/213/1/DissB_7139.pdf.
Rubas W, Jezyk N, Grass GM. Comparison of the permeability characteristics of a human colonic epithelial (Caco-2) cell line to colon of rabbit, monkey, and dog intestine and human drug absorption. Pharm Res. 1993;10:113–8.
Shugarts S, Benet L. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res. 2009;26:2039–54.
Christians U. Transport proteins and intestinal metabolism; P-glycoprotein and cytochrome P450 (CYP)-3A. Ther Drug Monit. 2004;26:104–6.
Szarka CE, Pleiffer GR, Hum ST, Everley LC, Baishem AM, Moore DF, et al. Glutathione S-transferase activity and glutathione s-transferase μ expression in subjects with risk for colorectal cancer. Cancer Res. 1995;55:2789–93.
https://www.fda.gov/downloads/Drugs/Guidances/ucm070246.pdf.
Zhao Y, Le J, Abraham MH, Hersey A, Eddershaw PJ, Luscombe CN, et al. Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure activity-relationship (QSAR) with the Abraham descriptors. J Pharm Sci. 2001;90:749–83.
Shirasaka Y, Kuraoka E, Spahn-Langguth H, Nakanishi T, Langguth P, Tamai I. Species difference in the effect of grapefruit juice on intestinal absorption of Talinolol between human and rat. J Pharmacol Exp Ther. 2010;332:181–9.
Bock U, Flototto T, Haltner E. Validation of cell culture models for the intestine and the blood-brain barrier and comparison of drug permeation. ALTEX. 2004;21(Suppl 3):57–64.
Zaki NM, Artursson P, Bergström C. A modified physiological BCS for prediction of intestinal absorption in drug discovery. Mol Pharm. 2010;7:1478–87.
Kiela PR, Ghishan FK. Physiology of intestinal absorption and secretion. Best Pract Res Clin Gastroenterol. 2016;30:145–59.
Crawley SW, Mooseker MS, Tyska MJ. Shaping the intestinal brush border destruction by enterohemorrhagic Escherchia coli (EHEC): new insights from organoid culture. J Cell Biol. 2014;207:441–51.
Holmes R, Lobley RW. Intestinal brush border revisited. Gut. 1998;30:1667–78.
Tyska MJ. Brush border destruction by enterohemorrhagic Escherchia coli (EHEC): new insights from organoid culture. Cell Mol Gastroenterol Hepatol. 2016;2:7–8.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89.
Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81.
Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickma JJ. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015;20:107–26.
Balimane PV, Han Y-H, Chong S. Current industrial practices of assessing permeability and p-glycoprotein interaction. AAPS J. 2006;8:E1–E13.
Hersman EM, Bumpus NN. A targeted proteomics approach for profiling murine cytochrome P450 expressions. J Pharmacol Exp Ther. 2014;349:221–8.
US FDA. Guidance for industry drug interaction studies — study design, data analysis, implications for dosing, and labeling recommendations. 2006. https://www.fda.gov/OHRMS/DOCKETS/98fr/06d-0344-gdl0001.pdf.
DiMarco RL, Hunt DR, Dewi RE, Heilshorn SC. Improvement of paracellular transport in the Caco-2 drug screening model using protein-engineered substrates. Biomaterials. 2017;129:152–62.
Cummins CL, Mangravite LM, Benet LZ. Characterizing the expression of CYP3A4 and efflux transporters (P-gp, MRP1, and MRP2) in CYP3A4-transfected Caco-2 cells after induction with sodium butyrate and the phorbol ester 12-O-tetradecanoylphorbol-13 acetate. Pharm Res. 2001;18:1102–9.
Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ramme AP, Hubner J, et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15:2688–99.
Car D, Ayehunie S, Davies A, Duckworth C, French S, Hall N, et al. Towards better modeling and mechanistic biomarkers for drug-induced gastrointestinal injury. Pharmacol Ther. 2017;172:181–94.
Eaton AD, Zimmermann Delaney CB, Hurley BP. Primary human polarized small intestinal epithelial barriers respond differently to a hazardous and an innocuous protein. Food Chem Toxicol. 2017;106:70–7.
Maldonado-Conteras A, Birtley JR, Boll E, Zhao Y, Mumy KL, Toscano J, et al. Shigella depends on SepA to destabilize the intestinal epithelial integrity via cofilin activation. Gut Microbes. 2017;8:544–60.
Anselmo AC, McHugh KJ, Webster J, Langer R, Jaklenec A. Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Adv Mater. 2016;28:9486–90.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayehunie, S., Landry, T., Stevens, Z. et al. Human Primary Cell-Based Organotypic Microtissues for Modeling Small Intestinal Drug Absorption. Pharm Res 35, 72 (2018). https://doi.org/10.1007/s11095-018-2362-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2362-0