Abstract
Purpose
The purpose of the research described herein was to develop a kinetic model for quantifying the effects of conditional and compositional variations on non-covalent polymorphic and covalent chemical transformations of gabapentin.
Methods
Kinetic models that describe the relationship between polymorphs and degradation product in a series of sequential or parallel steps were devised based on analysis of the resultant concentration time profiles. Model parameters were estimated using non-linear regression and Bayesian methods and evaluated in terms of their quantitative relationship to compositional and conditional variations.
Results
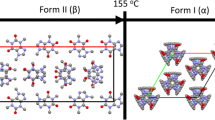
The model was constructed in which co-milling gabapentin with excipients determined three physically-initial concentrations (II0*, II0 and III0) and one chemically-initial concentration (lactam0). For chemical transitions, no humidity effect was present but the catalytic effects of excipients on the conversion of II and III➔lactam were observed. For physical transition, excipient primarily influenced the physical state transition of III➔II through its ability to interact with humidity.
Conclusions
This model was shown to be robust to quantitatively account for the effects of temperature, humidity and excipient on rate constants associated with kinetics for each physical and chemical transition.









Similar content being viewed by others
Abbreviations
- II0 :
-
Initial concentration of gabapentin Form II
- II0 * :
-
Estimated initial concentration of physically-disordered gabapentin Form II
- III0 :
-
Initial concentration of gabapentin Form III
- II➔III:
-
Physical state transition of gabapentin Form II➔III
- II*➔II:
-
Physical state transition of physically-disordered gabapentin Form II*➔II
- III➔II:
-
Physical state transition of gabapentin Form III➔II
- II➔lactam:
-
Chemical degradation of gabapentin Form II➔lactam
- II*➔lactam:
-
Chemical degradation of physically-disordered gabapentin Form II*➔lactam
- III➔lactam:
-
Chemical degradation of gabapentin Form III➔lactam
- AMASTK:
-
R-language based Advanced Modeling and Simulation Tool Kit
- β:
-
Humidity sensitivity term in modified Arrhenius equation
- 13C CP/MAS NMR:
-
13C cross-polarization/magic angle spinning nuclear magnetic resonance
- CaHPO4 :
-
Dibasic calcium phosphate dihydrate
- Form II or II:
-
Gabapentin Form II
- Form II* or II*:
-
Physically-disordered gabapentin Form II
- Form III or III:
-
Gabapentin Form III
- 1H T1 :
-
Proton relaxation time in laboratory frame
- HPC:
-
Hydroxy propyl cellulose
- HPLC:
-
High-performance liquid chromatography
- k1 :
-
Estimated rate constant for conversion of II*➔II
- k2 :
-
Estimated rate constant for rapid conversion of II*➔lactam
- k3 :
-
Estimated rate constant for the conversion of II➔lactam
- k4 :
-
Estimated rate constant for the conversion of III➔lactam
- k5 :
-
Estimated rate constant for polymorphic transformation of III➔II
- lactam or L:
-
Gabapentin-lactam
- Lactam0 or L0 :
-
Initial concentration of gabapentin-lactam
- MCMC:
-
Markov Chain Monte Carlo
- SiO2 :
-
Colloidal silicon dioxide
References
Dempah KE, Barich DH, Kaushal AM, Zong Z, Desai SD, Suryanarayanan R, et al. Investigating gabapentin polymorphism using solid-state NMR spectroscopy. AAPS PharmSciTech. 2013;14(1):19–28.
Lin SY, Hsu CH, Ke WT. Solid-state transformation of different gabapentin polymorphs upon milling and co-milling. Int J Pharm. 2010;396(1–2):83–90.
Zong Z, Desai SD, Kaushal A, Barich D, Huang H-S, Munson E, et al. The stabilizing effect of moisture on the solid-state degradation of gabapentin. AAPS PharmSciTech. 2011;12(3):924–31.
Reece HA, Levendis DC. Polymorphs of gabapentin. Acta Crystallogr Sect C: Cryst Struct Commun. 2008;C64(3):o105–8.
Zong Z, Qiu J, Tinmanee R, Kirsch L. Kinetic model for solid-state degradation of gabapentin. J Pharm Sci. 2012;101(6):2123–33.
Tinmanee R, Larsen S, Morris K, Kirsch L. Quantification of gabapentin polymorphs in gabapentin/excipient mixtures using solid state 13C NMR spectroscopy and X-ray powder diffraction. J Pharm Biomed Anal. 2017;146(29–36).
Braga D, Grepioni F, Maini L, Rubini K, Polito M, Brescello R, et al. Polymorphic gabapentin: thermal behaviour, reactivity and interconversion of forms in solution and solid-state. New J Chem. 2008;32(10):1788–95.
Soetaert K, Petzoldt T, Setzer W. Solving differential equations in R: Package deSolve. J Stat Softw. 2010;33(9):1–25.
Soetaert K, Petzoldt T. Inverse modelling, sensitivity and Monte Carlo analysis in R using package FME. J Stat Softw. 2010;33(3):1–28.
Blau G, Lasinski M, Orcun S, Hsu S-H, Caruthers J, Delgass N, et al. High fidelity mathematical model building with experimental data: A Bayesian approach. Comput Chem Eng. 2008;32(4–5):971–89.
Nash J. On best practice optimization methods in R. J Stat Softw. 2014;60(2):1–14.
Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E. Equation of state calculations by fast computing machines. J Chem Phys. 1953;21(6):1087–92.
Hastings WK. Monte Carlo sampling methods using Markov chains and their applications. Biometrika. 1970;57(1):97–109.
Brooks SP. Bayesian computation: a statistical revolution. Philos Trans A Math Phys Eng Sci. 2003;361(1813):2681–97.
Hao YJ, Tanaka T. Role of the contact points between particles on the reactivity of solid. Can J Chem Eng. 1988;66(5):761–6.
Qiu Z, Stowell JG, Cao W, Morris KR, Byrn SR, Carvajal MT. Effect of milling and compression on the solid-state maillard reaction. J Pharm Sci. 2005;94(11):2568–80.
Qiu Z, Stowell JG, Morris KR, Byrn SR, Pinal R. Kinetic study of the Maillard reaction between metoclopramide hydrochloride and lactose. Int J Pharm. 2005;303(1–2):20–30.
Zografi G. States of water associated with solids. Drug Dev Ind Pharm. 1988;14(14):1905–26.
Thakral NK, Ragoonanan V, Suryanarayanan R. Quantification, mechanism, and mitigation of active ingredient phase transformation in tablets. Mol Pharm. 2013;10(8):3128–36.
Yoshinari T, Forbes RT, York P, Kawashima Y. Moisture induced polymorphic transition of mannitol and its morphological transformation. Int J Pharm. 2002;247(1–2):69–77.
Acknowledgments and Disclosures
Special thanks are given to the University of Iowa Central Nuclear Magnetic Resonance Facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Tony Zhou and Tonglei Li
Rights and permissions
About this article
Cite this article
Tinmanee, R., Stamatis, S.D., Ueyama, E. et al. Polymorphic and Covalent Transformations of Gabapentin in Binary Excipient Mixtures after Milling-Induced Stress. Pharm Res 35, 39 (2018). https://doi.org/10.1007/s11095-017-2285-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-017-2285-1