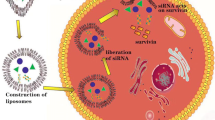
Abstract
Purpose
We have developed and evaluated novel peptide-targeted gemini surfactant-based lipoplexes designed for melanoma gene therapy.
Methods
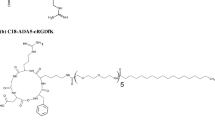
Integrin receptor targeting peptide, cyclic-arginylglycylaspartic acid (cRGD), was either chemically coupled to a gemini surfactant backbone or physically co-formulated with lipoplexes. Several formulations and transfection techniques were developed. Transfection efficiency and cellular toxicity of the lipoplexes were evaluated in an in vitro human melanoma model. Physicochemical properties were examined using dynamic light scattering, zeta-potential, and small-angle X-ray scattering measurements.
Results
RGD-modified gemini surfactant based lipoplexes showed significant enhancement in gene transfection activity in A375 cell lines compared to the standard non-targeted formulation, especially when RGD was chemically conjugated to the gemini surfactant (RGD-G). The RGD had no effect on the cell toxicity profile of the lipoplex systems. Targeting specificity was confirmed by using an excess of free RGD and negative control peptide (RAD) and was demonstrated by using normal human epidermal keratinocytes. Physicochemical characterization showed that all nanoparticles were in the optimal size range for cellular uptake and there were no significant differences between RGD-modified and standard lipoplexes.
Conclusions
These findings indicate the potential of RGD-modified gemini surfactant-based lipoplexes for use in melanoma gene therapy as an alternative to conventional chemotherapy.





Similar content being viewed by others
Abbreviations
- cRGD:
-
Cyclic-arginylglycylaspartic acid
- DOPE:
-
Phosphatidylethanolamine
- GFP:
-
Green fluorescent protein
- IFN-γ:
-
Interferon-gamma
- SAXS:
-
Small-angle X-ray scattering
References
Gershenwald JE, Giacco GG, Lee JE. Cutaneous Melanoma. 60 Years of Survival Outcomes at The University of Texas MD Anderson Cancer Center: Springer; 2013. p. 153–65.
Viola JR, Rafael DF, Wagner E, Besch R, Ogris M. Gene therapy for advanced melanoma: selective targeting and therapeutic nucleic acids. J Drug Deliv. 2013;2013. doi:10.1155/2013/897348.
Gene Therapy Clinical Trials Worldwide: John Wiley and Sons Ltd. The Journal of Gene Medicine Clinical Trial site. http://www.wiley.com//legacy/wileychi/genmed/clinical/ (2016). Accessed 25 April 2012.
Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. OncoImmunology. 2015;5(1):e1115641.
Bombelli C, Giansanti L, Luciani P, Mancini G. Gemini surfactant based carriers in gene and drug delivery. Curr Med Chem. 2009;16(2):171–83.
Wettig SD, Verrall RE, Foldvari M. Gemini surfactants: a new family of building blocks for non-viral gene delivery systems. Current Gene Ther. 2008;8(1):9–23.
Badea I, Verrall R, Baca-Estrada M, Tikoo S, Rosenberg A, Kumar P, et al. In vivo cutaneous interferon-γ gene delivery using novel dicationic (gemini) surfactant–plasmid complexes. J Gene Med. 2005;7(9):1200–14.
Donkuru MD, Wettig SD, Verrall RE, Badea I, Foldvari M. Designing pH-sensitive gemini nanoparticles for non-viral gene delivery into keratinocytes. J Mater Chem. 2012;22:6232–44.
Yang P, Singh J, Wettig S, Foldvari M, Verrall RE, Badea I. Enhanced gene expression in epithelial cells transfected with amino acid-substituted gemini nanoparticles. Eur J Pharm Biopharm. 2010;75(3):311–20.
Badea I. Gemini cationic surfactant-based delivery systems for non-invasive cutaneous gene therapy: University of Saskatchewan; 2006.
Badea I, Virtanen C, Verrall R, Rosenberg A, Foldvari M. Effect of topical interferon-γ gene therapy using gemini nanoparticles on pathophysiological markers of cutaneous scleroderma in tsk/+ mice. Gene Ther. 2012;19(10):978–87.
Singh J, Michel D, Chitanda JM, Verrall RE, Badea I. Evaluation of cellular uptake and intracellular trafficking as determining factors of gene expression for amino acid-substituted gemini surfactant-based DNA nanoparticles. J Nanobiotechnol. 2012;10(1):7.
Al-Dulaymi MA, Chitanda JM, Mohammed-Saeid W, Araghi HY, Verrall RE, Grochulski P, et al. Di-peptide-modified Gemini surfactants as Gene delivery vectors: exploring the role of the alkyl tail in their physicochemical behavior and biological activity. AAPS J. 2016:1–14.
Singh J, Michel D, Getson HM, Chitanda JM, Verrall RE, Badea I. Development of amino acid substituted gemini surfactant-based mucoadhesive gene delivery systems for potential use as noninvasive vaginal genetic vaccination. Nanomedicine. 2015;10(3):405–17.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87.
Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31(1):485–516.
Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer. 2004;90(3):561–5.
Tucker GC. Integrins: molecular targets in cancer therapy. Curr Oncol Rep. 2006;8(2):96–103.
Eble JA, Haier J. Integrins in cancer treatment. Curr Cancer Drug Targets. 2006;6(2):89–105.
Natali P, Bartolazzi A, Cavaliere R, Bigotti A, Nicotra M. Integrin expression in cutaneous malignant melanoma: association of the α3/β1 heterodimer with tumor progression. Int J Cancer. 1993;54(1):68–72.
Hieken TJ, Ronan SG, Farolan M, Shilkaitis AL, Kim DK, Gupta TKD. Beta1 integrin expression in malignant melanoma predicts occult lymph node metastases. Surgery. 1995;118(4):669–75.
Van Belle PA, Elenitsas R, Satyamoorthy K, Wolfe JT, Guerry D, Schuchter L, et al. Progression-related expression of β3 integrin in melanomas and nevi. Hum Pathol. 1999;30(5):562–7.
Harvie P, Dutzar B, Galbraith T, Cudmore S, O'Mahony D, Anklesaria P, et al. Targeting of lipid-protamine-DNA (LPD) lipopolyplexes using RGD motifs. J Liposome Res. 2003;13(3–4):231–47.
Shi W, Bartlett JS. RGD inclusion in VP3 provides adeno-associated virus type 2 (AAV2)-based vectors with a heparan sulfate-independent cell entry mechanism. Mol Ther. 2003;7(4):515–25.
Eto Y, Gao JQ, Sekiguchi F, Kurachi S, Katayama K, Maeda M, et al. PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability. J Gene Med. 2005;7(5):604–12.
Mohammed-Saeid W, Buse J, Badea I, Verrall R, El-Aneed A. Mass spectrometric analysis of amino acid/di-peptide modified gemini surfactants used as gene delivery agents: establishment of a universal mass spectrometric fingerprint. Int J Mass Spectrom. 2012;309:182–91.
Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377(Pt 1):159.
Resina S, Prevot P, Thierry AR. Physico-chemical characteristics of lipoplexes influence cell uptake mechanisms and transfection efficacy. PLoS One. 2009;4(6):e6058.
Dubey PK, Mishra V, Jain S, Mahor S, Vyas S. Liposomes modified with cyclic RGD peptide for tumor targeting. J Drug Target. 2004;12(5):257–64.
Mulder WJ, Strijkers GJ, Habets JW, Bleeker EJ, van der Schaft DW, Storm G, et al. MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. FASEB J. 2005;19(14):2008–10.
Schiffelers RM, Koning GA, ten Hagen TL, Fens MH, Schraa AJ, Janssen AP, et al. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J Control Release. 2003;91(1):115–22.
Adams JC, Watt FM. Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol. 1991;115(3):829–41.
Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, et al. Keratinocytes in human wounds express αvβ6 integrin. J Investig Dermatol. 1996;106(1):42–8.
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22.
Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci. 2008;105(33):11613–8.
Kunath K, Merdan T, Hegener O, Häberlein H, Kissel T. Integrin targeting using RGD-PEI conjugates for in vitro gene transfer. J Gene Med. 2003;5(7):588–99.
Xiong XB, Huang Y, WL LU, Zhang X, Zhang H, Nagai T, et al. Intracellular delivery of doxorubicin with RGD-modified sterically stabilized liposomes for an improved antitumor efficacy: in vitro and in vivo. J Pharm Sci. 2005;94(8):1782–93.
Panetti T, McKeown-Longo P. The alpha v beta 5 integrin receptor regulates receptor-mediated endocytosis of vitronectin. J Biol Chem. 1993;268(16):11492–5.
Foldvari M, Badea I, Wettig S, Verrall R, Bagonluri M. Structural characterization of novel gemini non-viral DNA delivery systems for cutaneous gene therapy. J Exp Nanosci. 2006;1(2):165–76.
Solomon DE. An in vitro examination of an extracellular matrix scaffold for use in wound healing. Int J Exp Pathol. 2002;83(5):209–16.
Acknowledgments and Disclosures
The authors report no conflicts of interest. The authors are grateful for financial support from NSERC and SHRF to conduct this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Figure S-1
(DOCX 66 kb)
Figure S-2
(DOCX 72 kb)
Rights and permissions
About this article
Cite this article
Mohammed-Saeid, W., Chitanda, J., Al-Dulaymi, M. et al. Design and Evaluation of RGD-Modified Gemini Surfactant-Based Lipoplexes for Targeted Gene Therapy in Melanoma Model. Pharm Res 34, 1886–1896 (2017). https://doi.org/10.1007/s11095-017-2197-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2197-0