Abstract
Purpose
To summarize the microstructure – property relationship of pharmaceutical tablets and approaches to improve tablet properties through tablet microstructure engineering.
Method
The main topics reviewed here include: 1) influence of material properties and manufacturing process parameters on the evolution of tablet microstructure; 2) impact of tablet structure on tablet properties; 3) assessment of tablet microstructure; 4) development and engineering of tablet microstructure.
Results
Microstructure plays a decisive role on important pharmaceutical properties of a tablet, such as disintegration, drug release, and mechanical strength. Useful information on mechanical properties of a powder can be obtained from analyzing tablet porosity—pressure data. When helium pycnometry fails to accurately measure true density of a water-containing powder, non-linear regression of tablet density—pressure data is a useful alternative method. A component that is more uniformly distributed in a tablet generally exerts more influence on the overall tablet properties.
Conclusion
During formulation development, it is highly recommended to examine the relationship between any property of interest and tablet porosity when possible. Tablet microstructure can be engineered by judicious selection of formulation composition, including the use of the optimum solid form of the drug and appropriate type and amount of excipients, and controlling manufacturing process.





Similar content being viewed by others
References
Jivraj M, Martini LG, Thomson CM. An overview of the different excipients useful for the direct compression of tablets. Pharm Sci Technol Today. 2000;3(2):58–63.
USP 39-NF 34 <1151> Pharmaceutical dosage forms. 2016.
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48:27–42.
Vippagunta SR, Brittain HG, Grant DJW. Crystalline solids. Adv Drug Deliv Rev. 2001;48(1):3–26.
Brittain HG, Grant DJW. Effects of polymorphism and solid-state solvation on solubility and dissolution rate. In: Brittain HG, editor. Polymorphism in pharmaceutical solids. New York: Marcel Dekker, Inc.; 1999. p. 279–330.
Morris KR, Fakes MG, Thakur AB, Newman AW, Singh AK, Venit JJ, et al. An integrated approach to the selection of optimal salt form for a new drug candidate. Int J Pharm. 1994;105(3):209–17.
Heinrich SP, Wermuth CG. Handbook of pharmaceutical salts; properties, selection, and use. Wiley-VCH; 2002.
Vishweshwar P, McMahon JA, Bis JA, Zaworotko MJ. Pharmaceutical co-crystals. J Pharm Sci. 2006;95(3):499–516.
Hou H, Sun CC. Quantifying effects of particulate properties on powder flow properties using a ring shear tester. J Pharm Sci. 2008;97(9):4030–9.
Sun CC, Grant DJW. Effects of initial particle size on the tableting properties of L-lysine monohydrochloride dihydrate powder. Int J Pharm. 2001;215:221–8.
Sun CC, Grant DJW. Influence of crystal shape on the tableting performance of L-lysine monohydrochloride dihydrate. J Pharm Sci. 2001;90:567–77.
Sun CC. Materials Science Tetrahedron—a useful tool for pharmaceutical research and development. J Pharm Sci. 2009;98:1671–87.
Sun CC. Critical roles of porosity in tableting properties characterization and solids formulation development. Am Pharm Rev. 2005;8(6):102, 104–107.
Desai PM, Liew CV, Heng PWS. Review of disintegrants and the disintegration phenomena. J Pharm Sci.
Sunada H, Bi Y. Preparation, evaluation and optimization of rapidly disintegrating tablets. Powder Technol. 2002;122(2–3):188–98.
Hancock BC, Colvi JT, Mullarney MP, Zinchuk AV. The relative densities of pharmaceutical powders, blends, dry granulations, and immediate-release tablets. Pharm Technol. 2003;27:64–80.
Gong X, Sun CC. A new tablet brittleness index. Eur J Pharm Biopharm. 2015;93:260–6.
Gong X, Chang S-Y, Osei-Yeboah F, Paul S, Perumalla SR, Shi L, et al. Dependence of tablet brittleness on tensile strength and porosity. Int J Pharm. 2015;493(1–2):208–13.
Knudsen FP. Effect of porosity on Young’s modulus of alumina. J Am Ceram Soc. 1962;45:94–5.
Spriggs RM. Expression for effect of porosity on elastic modulus of polycrystalline refractory material, particularly aluminum, oxide. J Am Ceram Soc. 1961;44:628–9.
Patel S, Sun CC. Macroindentation hardness measurement—modernization and applications. Int J Pharm. 2016;506(1–2):262–7.
Ryshkewitch E. Compression strength of porous sintered alumina and zirconia. J Am Ceram Soc. 1953;36(65–68).
Sun CC. A material-sparing method for simultaneous determination of true density and powder compaction properties - aspartame as an example. Int J Pharm. 2006;326:94–9.
Osei-Yeboah F, Sun CC. Validation and applications of an expedited tablet friability method. Int J Pharm. 2015;484(1–2):146–55.
Modi SR, Dantuluri AK, Perumalla SR, Sun CC, Bansal AK. Effect of crystal habit on intrinsic dissolution behavior of celecoxib due to differential wettability. Cryst Growth Des. 2014;14(10):5283–92.
Heng JYY, Bismarck A, Lee AF, Wilson K, Williams DR. Anisotropic surface chemistry of aspirin crystals. J Pharm Sci. 2007;96(8):2134–44.
Heng JYY, Bismarck A, Lee AF, Wilson K, Williams DR. Anisotropic surface energetics and wettability of macroscopic form I paracetamol crystals. Langmuir. 2006;22(6):2760–9.
Smrčka D, Dohnal J, Stěpánek F. Dissolution and disintegration kinetics of high-active pharmaceutical granules produced at laboratory and manufacturing scale. Eur J Pharm Biopharm. 2016. doi:10.1016/j.ejpb.2016.1004.1005.
Çelik M. Overview of compaction data analysis techniques. Drug Dev Ind Pharm. 1992;18(6–7):767–810.
Heckel RW. Density-pressure relations in powder compaction. Trans Metall Soc AIME. 1961;221:671–5.
Heckel RW. An analysis of powder compaction phenomena. Trans Metall Soc AIME. 1961;221:1001–8.
Kuentz M, Leuenberger H. Pressure susceptibility of polymer tablets as a critical property: a modified Heckel equation. J Pharm Sci. 1999;88(2):174–9.
Hersey JA, Rees JE. Deformation of particles during briqueting. Nat (London), Phys Sci. 1971;230(12):96.
Rue PJ, Rees JE. Limitations of the Heckel relation for predicting powder compaction mechanisms. J Pharm Pharmacol. 1978;30(1):642–3.
Sun CC. Thermal expansion of organic crystals and precision of calculated crystal density: a survey of Cambridge Crystal Database. J Pharm Sci. 2007;96:1043–52.
Viana M, Jouannin P, Pontier C, Chulia D. About pycnometric density measurements. Talanta. 2002;57(3):583–93.
Sun CC. A novel method for deriving true density of pharmaceutical solids including hydrates and water-containing powders. J Pharm Sci. 2004;93(3):646–53.
Sun CC. True density of microcrystalline cellulose. J Pharm Sci. 2005;94:2132–334.
Sun CC. Mechanism of moisture induced variations in true density and compaction properties of microcrystalline cellulose. Int J Pharm. 2008;346:93–101.
Paul S, Chang S-Y, Sun CC. The phenomenon of tablet flashing—its impact on tableting data analysis and a method to eliminate it. Powder Technol. 2016. Under review.
Envelope density measurements by micromeritics’ GeoPyc 1360 and other methods micromeritics application Note #97. Available from: http://www.micromeritics.com/Repository/Files/ap97.pdf.
Geopyc 1360 envelop density analyzer. Micromeritics. Available from: http://www.micromeritics.com/repository/files/geopyc_1360_reg_and_tap.pdf.
Allesø M, Holm R, Holm P. Roller compaction scale-up using roll width as scale factor and laser-based determined ribbon porosity as critical material attribute. Eur J Pharm Sci. 2016;87:69–78.
Khorasani M, Amigo JM, Sonnergaard J, Olsen P, Bertelsen P, Rantanen J. Visualization and prediction of porosity in roller compacted ribbons with near-infrared chemical imaging (NIR-CI). J Pharm Biomed Anal. 2015;109:11–7.
Nordström J, Persson A-S, Lazorova L, Frenning G, Alderborn G. The degree of compression of spherical granular solids controls the evolution of microstructure and bond probability during compaction. Int J Pharm. 2013;442(1–2):3–12.
Khorasani M, Amigo JM, Bertelsen P, Sun CC, Rantanen J. Process optimization of dry granulation based tableting line: extracting physical material characteristics from granules, ribbons and tablets using near-IR (NIR) spectroscopic measurement. Powder Technol.
Van Brakel J, Modrý S, Svatá M. Mercury porosimetry: state of the art. Powder Technol. 1981;29(1):1–12.
Diamond S. Mercury porosimetry: an inappropriate method for the measurement of pore size distributions in cement-based materials. Cem Concr Res. 2000;30(10):1517–25.
Ridgway CJ, Schoelkopf J, Matthews GP, Gane PA, James PW. The effects of void geometry and contact angle on the absorption of liquids into porous calcium carbonate structures. J Colloid Interface Sci. 2001;239:417–31.
Ridgway CJ, Gane PAC, Schoelkopf J. Effect of capillary element aspect ratio on the dynamic imbibition within porous networks. J Colloid Interface Sci. 2002;252(2):373–82.
Liu G, Zhang M, Ridgway C, Gane P. Spontaneous inertial imbibition in porous media using a fractal representation of pore wall rugosity. Transp Porous Media. 2014;104(1):231–51.
Schölkopf J, Gane PAC, Ridgway CJ, Matthews GP. Influence of inertia on liquid absorption into paper coating structures. Nord Pulp Pap Res J. 2000;15:422–30.
Ridgway CJ, Gane PAC, Schoelkopf J. Modified calcium carbonate coatings with rapid absorption and extensive liquid uptake capacity. Colloids Surf A Physicochem Eng Asp. 2004;236(1–3):91–102.
Liu G, Zhang M, Ridgway C, Gane P. Pore wall rugosity: the role of extended wetting contact line length during spontaneous liquid imbibition in porous media. Colloids Surf A Physicochem Eng Asp. 2014;443:286–95.
Shi L, Feng Y, Sun CC. Roles of granule size in over-granulation during high shear wet granulation. J Pharm Sci. 2010;99(8):3322–5.
Shi L, Feng Y, Sun CC. Origin of profound changes in powder properties during wetting and nucleation stages of high shear wet granulation. Powder Technol. 2011;208:663–8.
Shi L, Feng Y, Sun CC. Massing in high shear wet granulation can simultaneously improve powder flow and deteriorate powder compaction: a double-edged sword. Eur J Pharm Sci. 2011;43:50–6.
Willi AK. X-ray computed tomography. Phys Med Biol. 2006;51(13):R29.
Sinka IC, Burch SF, Tweed JH, Cunningham JC. Measurement of density variations in tablets using X-ray computed tomography. Int J Pharm. 2004;271(1–2):215–24.
Farber L, Tardos G, Michaels JN. Use of X-ray tomography to study the porosity and morphology of granules. Powder Technol. 2003;132(1):57–63.
Wu CY, Hancock BC, Mills A, Bentham AC, Best SM, Elliott JA. Numerical and experimental investigation of capping mechanisms during pharmaceutical tablet compaction. Powder Technol. 2008;181:121–9.
Akseli I, Abebe A, Sprockel O, Cuitiño AM. Mechanistic characterization of bilayer tablet formulations. Powder Technol. 2013;236:30–6.
Hancock BC, Mullarney MP. X-ray microtomography of solid dosage forms. Pharm Technol. 2005;29(44):92–100.
Zeitler JA, Shen Y, Baker C, Taday PF, Pepper M, Rades T. Analysis of coating structures and interfaces in solid oral dosage forms by three dimensional terahertz pulsed imaging. J Pharm Sci. 2007;96(2):330–40.
Krok A, García-Triñanes P, Peciar M, Wu C-Y. Finite element analysis of thermomechanical behaviour of powders during tabletting. Chem Eng Res Des.
Mazel V, Diarra H, Busignies V, Tchoreloff P. Evolution of the die-wall pressure during the compression of biconvex tablets: experimental results and comparison with FEM simulation. J Pharm Sci. 2015;104(12):4339–44.
Garner S, Ruiz E, Strong J, Zavaliangos A. Mechanisms of crack formation in die compacted powders during unloading and ejection: an experimental and modeling comparison between standard straight and tapered dies. Powder Technol. 2014;264:114–27.
Yohannes B, Gonzalez M, Abebe A, Sprockel O, Nikfar F, Kiang S, et al. Evolution of the microstructure during the process of consolidation and bonding in soft granular solids. Int J Pharm. 2016;503(1–2):68–77.
Ridgway CJ, Gane PAC. Dynamic absorption into simulated porous structures. Colloids Surf A Physicochem Eng Asp. 2002;206(1–3):217–39.
Bi YX, Sunada H, Yonezawa Y, Danjo K. Evaluation of rapidly disintegrating tablets prepared by a direct compression method. Drug Dev Ind Pharm. 1999;25(5):571–81.
Tye CK, Sun CC, Amidon GE. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J Pharm Sci. 2005;94(3):465–72.
Kendall K. The impossibility of comminuting small particles by compression. Nature (London). 1978;272(5655):710–1.
Garr JSM, Rubinstein MH. An investigation into the capping of paracetamol at increasing speeds of compression. Int J Pharm. 1991;72(2):117–22.
Akande OF, Rubinstein MH, Rowe PH, Ford JL. Effect of compression speeds on the compaction properties of a 1:1 paracetamol–microcrystalline cellulose mixture prepared by single compression and by combinations of pre-compression and main-compression. Int J Pharm. 1997;157(2):127–36.
Akseli I, Ladyzhynsky N, Katz J, He X. Development of predictive tools to assess capping tendency of tablet formulations. Powder Technol. 2013;236:139–48.
Sun CC, Grant DJW. Improved tableting properties of p-hydroxybenzoic acid by water of crystallization: a molecular insight. Pharm Res. 2004;21(2):382–6.
Malaj L, Censi R, Gashi Z, Di Martino P. Compression behaviour of anhydrous and hydrate forms of sodium naproxen. Int J Pharm. 2010;390(2):142–9.
Khomane KS, More PK, Raghavendra G, Bansal AK. Molecular understanding of the compaction behavior of indomethacin polymorphs. Mol Pharm. 2013;10(2):631–9.
Osei-Yeboah F, Feng Y, Sun CC. Evolution of structure and properties of granules containing microcrystalline cellulose and polyvinylpyrrolidone during high-shear wet granulation. J Pharm Sci. 2014;103(1):207–15.
Patel S, Dahiya S, Sun CC, Bansal AK. Understanding size enlargement and hardening of granules on tabletability of unlubricated granules prepared by dry granulation. J Pharm Sci. 2011;100:758–66.
Johansson B, Wikberg M, Ek R, Alderborn G. Compression behaviour and compactability of microcrystalline cellulose pellets in relationship to their pore structure and mechanical properties. Int J Pharm. 1995;117:57–73.
Nordström J, Alderborn G. The granule porosity controls the loss of compactibility for both dry- and wet-processed cellulose granules but at different rate. J Pharm Sci. 2015;104(6):2029–39.
Sun CC, Himmelspach MW. Reduced tabletability of roller compacted granules as a result of granule size enlargement. J Pharm Sci. 2006;95:200–6.
Osei-Yeboah F, Zhang M, Feng Y, Sun CC. A formulation strategy for solving the overgranulation problem in high shear wet granulation. J Pharm Sci. 2014;103(8):2434–40.
Sun CC. Decoding powder tabletability: roles of particle adhesion and plasticity. J Adhes Sci Technol. 2011;25(4–5):483–99.
Stauffer D, Aharony A. Introduction to percolation theory. London: Taylor and Francis; 1992.
Leuenberger H, Leu R. Formation of a tablet: a site and bond percolation phenomenon. J Pharm Sci. 1992;81(10):976–82.
Leuenberger H, Rohera BD, Haas C. Percolation theory - a novel approach to solid dosage form design. Int J Pharm. 1987;38:109–15.
Leuenberger H. The application of percolation theory in powder technology. Adv Powder Technol. 1999;10(4):323–52.
Shi L, Sun CC. Overcoming poor tabletability of pharmaceutical crystals by surface modification. Pharm Res. 2011;12:3248–55.
Shi L, Sun CC. Transforming powder mechanical properties by core/shell structure: compressible sand. J Pharm Sci. 2010;99(11):4458–62.
Osei-Yeboah F, Sun CC. Tabletability modulation through surface engineering. J Pharm Sci. 2015;104(8):2645–8.
Mehrotra A, Llusa M, Faqih A, Levin M, Muzzio FJ. Influence of shear intensity and total shear on properties of blends and tablets of lactose and cellulose lubricated with magnesium stearate. Int J Pharm. 2007;336(2):284–91.
Shah AC, Mlodozeniec AR. Mechanism of surface lubrication: influence of duration of lubricant - excipient mixing on processing characteristics of powders and properties of compressed tablets. J Pharm Sci. 1977;66:1377–82.
Wang J, Wen H, Desai D. Lubrication in tablet formulations. Eur J Pharm Biopharm. 2010;75(1):1–15.
Gupta AK, Mittal A, Jha K. Fast dissolving tablet-a review. Pharma Innov. 2012;1(1).
Fu Y, Yang S, Jeong SH, Kimura S, Park K. Orally fast disintegrating tablets: developments, technologies. Taste-Masking Clin Stud. 2004;21(6):44.
Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–7.
Yu DG, Zhu L-M, Branford-White CJ, Yang XL. Three-dimensional printing in pharmaceutics: promises and problems. J Pharm Sci. 2008;97(9):3666–90.
Khaled SA, Burley JC, Alexander MR, Roberts CJ. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int J Pharm. 2014;461(1–2):105–11.
ACKNOWLEDGMENTS AND DISCLOSURES
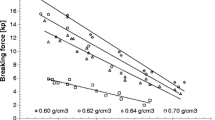
I thank Shubhajit Paul, Chenguang Wang, and Jiangnan Dun for useful comments during the preparation of this manuscript. Data used in Fig. 2 were collected by Shubhajit Paul.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, C.C. Microstructure of Tablet—Pharmaceutical Significance, Assessment, and Engineering. Pharm Res 34, 918–928 (2017). https://doi.org/10.1007/s11095-016-1989-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1989-y