Abstract
Purpose
To present a convenient screening method for evaluating additive effects on the renaturation of an acid-exposed monoclonal antibody (mAb).
Methods
The assay involves brief incubation of a mAb at acidic pH and subsequent neutralization in the absence or presence of additive to induce mainly aggregation. An increase in absorbance depicted aggregation. The recorded aggregation data traces were fitted with a nucleation-autocatalytic growth model for the extraction of kinetic parameters.
Results
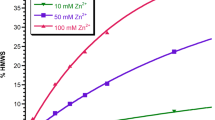
All kinetic data traces were fitted successfully with the selected model and the adjusted R square values were greater than 0.99. Trehalose had strongly stabilizing, proline mildly stabilizing and trimethylamine oxide had destabilizing effects on both the nucleation and growth phase of the reaction. Histidine was strongly stabilizing but was limited by its poor solubility.
Conclusion
The results demonstrate the suitability of the experimental mAb aggregation system and the nucleation-autocatalytic growth fit in the screening and quantification of additive effects on the renaturation of an acid-exposed mAb respectively. This will aid the investigation and derivation of quantitative structure-activity relationships of additive effects on mAb solubility.





Similar content being viewed by others
Abbreviations
- Bis-ANS:
-
4, 4′-Bis (1-anilinonaphthalene 8-sulfonate)
- ca.:
-
Circa
- DMSO:
-
Dimethyl sulfoxide
- DSF:
-
Differential Scanning Fluorimetry
- Eq.:
-
Equation
- HCl:
-
Hydrochloric acid
- HPLC:
-
High Pressure Liquid Chromatography
- HP-SEC:
-
High Performance–Size Exclusion Chromatography
- mAb:
-
Monoclonal antibody
- min:
-
Minutes
- NaOH:
-
Sodium hydroxide
- QSAR:
-
Quantitative Structure Activity Relationship
- s:
-
Seconds
- Tm :
-
(Apparent) Melting temperatures
- TMAO:
-
Trimethylamine N-oxide
References
Goswami S, Wang W, Arakawa T, Ohtake S. Developments and challenges for mAb-based therapeutics. Antibodies. 2013;2(3):452–500.
Southwell AL, Patterson PH. Antibody therapy in neurodegenerative disease. Rev Neurosci. 2010;21(4):273–87.
Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8(3):E501–7.
Vázquez-Rey M, Lang DA. Aggregates in monoclonal antibody manufacturing processes. Biotechnol Bioeng. 2011. p. 1494–508.
Cromwell MEM, Hilario E, Jacobson F. Protein aggregation and bioprocessing. AAPS J. 2006. p. E572–9.
Filipe V, Poole R, Oladunjoye O, Braeckmans K, Jiskoot W. Detection and characterization of subvisible aggregates of monoclonal IgG in serum. Pharm Res. 2012;29(8):2202–12.
Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm. 2005;289(1–2):1–30.
Lilie H, Schwarz E, Rudolph R. Advances in refolding of proteins produced in E. coli. Curr Opin Biotechnol. 1998;9(5):497–501.
Philo JS, Arakawa T. Mechanisms of protein aggregation. Curr Pharm Biotechnol. 2009;10(4):348–51.
Kamerzell TJ, Esfandiary R, Joshi SB, Middaugh CR, Volkin DB. Protein-excipient interactions: mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev Elsevier BV. 2011;63(13):1118–59.
Liu HF, Ma J, Winter C, Bayer R. Recovery and purification process development for monoclonal antibody production. mAbs. 2010. p. 480–99.
Dong X-Y, Huang Y, Sun Y. Refolding kinetics of denatured-reduced lysozyme in the presence of folding aids. J Biotechnol. 2004;114(1–2):135–42.
Goldberg ME, Rudolph R, Jaenicke R. A kinetic study of the competition between renaturation and aggregation during the refolding of denatured-reduced egg white lysozyme. Biochemistry. 1991;30(11):2790–7.
Hevehan DL, De Bernardez Clark E. Oxidative renaturation of lysozyme at high concentrations. Biotechnol Bioeng. 1997;54(3):221–30.
Brummitt RK, Nesta DP, Chang L, Chase SF, Laue TM, Roberts CJ. Nonnative aggregation of an IgG1 antibody in acidic conditions: part 1. Unfolding, colloidal interactions, and formation of high-molecular-weight aggregates. J Pharm Sci. 2011;100(6):2087–103.
Ishikawa T, Ito T, Endo R, Nakagawa K, Sawa E, Wakamatsu K. Influence of pH on heat-induced aggregation and degradation of therapeutic monoclonal antibodies. Biol Pharm Bull. 2010;33(8):1413–7.
Hari SB, Lau H, Razinkov VI, Chen S, Latypov RF. Acid-induced aggregation of human monoclonal IgG1 and IgG2: molecular mechanism and the effect of solution composition. Biochemistry. 2010;49(43):9328–38.
Lewis J, Ju R, Kim A, Nail S. Kinetics of low pH induced aggregation of equine IgG. J Colloid Interface Sci. 1997;196(2):170–6.
Neergaard MS, Nielsen AD, Parshad H, Van De Weert M. Stability of monoclonal antibodies at high-concentration: head-to-head comparison of the IgG1 and IgG4 subclass. J Pharm Sci. 2014;103(1):115–27.
Filipe V, Kukrer B, Hawe A, Jiskoot W. Transient molten globules and metastable aggregates induced by brief exposure of a monoclonal IgG to Low pH. J Pharm Sci. 2012;101(7):2327–39.
Samra HS, He F. Advancements in high throughput biophysical technologies: applications for characterization and screening during early formulation development of monoclonal antibodies. Mol Pharm. 2012;9(4):696–707.
Kiefhaber T, Rudolph R, Kohler HH, Buchner J. Protein aggregation in vitro and in vivo: a quantitative model of the kinetic competition between folding and aggregation. Biotechnology (N Y). 1991;9(9):825–9.
Sabaté R, Gallardo M, Estelrich J. An autocatalytic reaction as a model for the kinetics of the aggregation of beta-amyloid. Biopolymers. 2003;71(2):190–5.
Morris AM, Watzky MA, Agar JN, Finke RG. Fitting neurological protein aggregation kinetic data via a 2-step, minimal/“Ockham’s razor” model: the Finke-Watzky mechanism of nucleation followed by autocatalytic surface growth. Biochemistry. 2008;47(8):2413–27.
Kamihira M, Naito A, Tuzi S, Nosaka AY, Saitô H. Conformational transitions and fibrillation mechanism of human calcitonin as studied by high-resolution solid-state 13C NMR. Protein Sci. 2000;9(5):867–77.
Borgia MB, Nickson AA, Clarke J, Hounslow MJ. A mechanistic model for amorphous protein aggregation of immunoglobulin-like domains. J Am Chem Soc. 2013;135(17):6456–64.
Stranks SD, Ecroyd H, Van Sluyter S, Waters EJ, Carver JA, von Smekal L. Model for amorphous aggregation processes. Phys Rev E Stat Nonlinear Soft Matter Phys. 2009;80(5 Pt 1):051907.
Brummitt RK, Nesta DP, Chang L, Kroetsch AM, Roberts CJ. Nonnative aggregation of an IgG1 antibody in acidic conditions, part 2: nucleation and growth kinetics with competing growth mechanisms. J Pharm Sci. 2011;100(6):2104–19.
Krishnan S, Raibekas AA. Multistep aggregation pathway of human interleukin-1 receptor antagonist: kinetic, structural, and morphological characterization. Biophys J Biophys Soc. 2009;96(1):199–208.
Dawson RMC, Elliot DC, Elliot WH, Jones KM. Data for biochemical research [Internet]. Oxford Science publication. 1986 [cited 2015 Aug 19]. Available from: http://www.sigmaaldrich.com/life-science/core-bioreagents/biological-buffers/learning-center/buffer-reference-center.html#citric
Ghosh R, Sharma S, Chattopadhyay K. Effect of arginine on protein aggregation studied by fluorescence correlation spectroscopy and other biophysical methods. Biochemistry. 2009;48(5):1135–43.
Menzen T, Friess W. High-throughput melting-temperature analysis of a monoclonal antibody by differential scanning fluorimetry in the presence of surfactants. J Pharm Sci. 2013;102(2):415–28.
Buchner J, Renner M, Lilie H, Hinz HJ, Jaenicke R, Kiefhabel T, et al. Alternatively folded states of an immunoglobulin. Biochemistry. 1991;30(28):6922–9.
Bam NB, Cleland JL, Randolph TW. Molten globule intermediate of recombinant human growth hormone: stabilization with surfactants. Biotechnol Prog. 1996;12(6):801–9.
Chaudhuri R, Cheng Y, Middaugh CR, Volkin DB. High-throughput biophysical analysis of protein therapeutics to examine interrelationships between aggregate formation and conformational stability. AAPS J. 2014;16(1):48–64.
Samuel D, Kumar TK, Ganesh G, Jayaraman G, Yang PW, Chang MM, et al. Proline inhibits aggregation during protein refolding. Protein Sci. 2000;9(2):344–52.
Zou Q, Bennion BJ, Daggett V, Murphy KP. The molecular mechanism of stabilization of proteins by TMAO and its ability to counteract the effects of urea. J Am Chem Soc. 2002;124(7):1192–202.
Scaramozzino F, Peterson DW, Farmer P, Gerig JT, Graves DJ, Lew J. TMAO promotes fibrillization and microtubule assembly activity in the C-terminal repeat region of tau. Biochemistry. 2006;45(11):3684–91.
Yam GHF, Wang K, Jhanji V, Choy KW, Baum L, Pang CP. In vitro amyloid aggregate forming ability of TGFBI mutants that cause corneal dystrophies. Investig Ophthalmol Vis Sci. 2012;53(9):5890–8.
Taneja S, Ahmad F. Increased thermal stability of proteins in the presence of amino acids. Biochem J. 1994;303(Pt 1):147–53.
Falconer RJ, Chan C, Hughes K, Munro TP. Stabilization of a monoclonal antibody during purification and formulation by addition of basic amino acid excipients. J Chem Technol Biotechnol. 2011;86(7):942–8.
ACKNOWLEDGMENTS AND DISCLOSURES
This research was funded by the Federal Ministry of Education and Research, Germany (BMBF), grant number 0315338A. The authors are grateful to Bernd Burghardt for preliminary data exploration and programming sessions in R statistical software. Thanks are due to René Handrick, Karen Schwab, Fabian Bickel, Albrecht Weber and Alba Signes Marrahi for gifts of mAb1 at various time points during the execution of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oyetayo, OO., Kiefer, H. Experimental Model System to Study pH Shift-Induced Aggregation of Monoclonal Antibodies Under Controlled Conditions. Pharm Res 33, 1359–1369 (2016). https://doi.org/10.1007/s11095-016-1878-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1878-4