Abstract
Purpose
To clarify the effects of pump pulsation and flow-through cell (FTC) dissolution system settings on the hydrodynamic properties and dissolution profiles of model formulations.
Methods
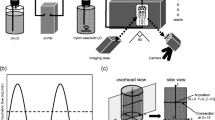
Two FTC systems with different cell temperature control mechanisms were used. Particle image velocimetry (PIV) was used to analyze the hydrodynamic properties of test solutions in the flow-through dissolution test cell. Two pulsation pumps (semi-sine, full-sine) and a non-pulsatile pump were used to study the effects of varied flows on the dissolution profiles of United States Pharmacopeia standard tablets.
Results
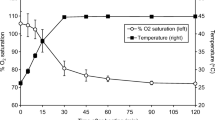
PIV analysis showed periodic changes in the aligned upward fluid flow throughout the dissolution cell that was designed to reduce the temperature gradient during pump pulsation (0.5 s/pulse). The maximum instantaneous flow from the semi-sine pump was higher than that of the full-sine pump under all conditions. The flow from the semi-sine wave pump showed faster dissolution of salicylic acid and prednisone tablets than those from other pumps. The semi-sine wave pump flow showed similar dissolution profiles in the two FTC systems.
Conclusions
Variations in instantaneous fluid flow caused by pump pulsation that meets the requirements of pharmacopoeias are a factor that affects the dissolution profiles of tablets in FTC systems.












Similar content being viewed by others
Abbreviations
- CFD:
-
Computational fluid dynamics
- EP:
-
European pharmacopoeia
- FTC:
-
Flow-through cell
- ID:
-
Inside diameter
- JP:
-
Japanese pharmacopoeia
- PIV:
-
Particle imaging velocimetry
- Tp :
-
Time phase of pump
- USP:
-
United States pharmacopeia
References
Fotaki N, Reppas C. The flow through cell methodology in the evaluation of intralumenal drug release characteristics. Dissolution Technol. 2005;12:17–21.
Moller H, Wirbitzki E. Regulatory aspects of modified release dosage forms: special cases of dissolution testing using the flow-through system. Boll Chim Farm. 1993;132(4):105–15.
D’Arcy DM, Liu B, Persoons T, Corrigan OI. Hydrodynamic complexity induced by the pulsing flow field in USP dissolution apparatus 4. Dissolution Technol. 2011;18(4):6–13.
Beyssac E, Lavigne J. Dissolution study of active pharmaceutical ingredients using the flow through apparatus USP 4. Dissolution Technol. 2005;5:23–5.
Kakhi M. Mathematical modeling of the fluid dynamics in the flow-through cell. Int J Pharm. 2009;376(1–2):22–40.
Langenbucher F, Benz D, Kurth W, Moller H, Otz M. Standardized flow-cell method as an alternative to existing pharmacopœial dissolution testing. Pharm Ind. 1989;51:1276–81.
Shiko G, Gladden LF, Sederman AJ, Connolly PC, Butler JM. MRI studies of the hydrodynamics in a USP 4 dissolution testing cell. J Pharm Sci. 2011;100(3):976–91.
Lerk CF, Zuurman K. The influence of pulsation on the dissolution rate measurements in column type apparatus. J Pharm Pharmacol. 1970;22(4):319–20.
Bielen N. Performance of USP calibrator tablets in flow-through cell apparatus. Int J Pharm. 2002;233(1–2):123–9.
Eaton JW, Tran D, Hauck WW, Stippler ES. Development of a performance verification test for USP apparatus 4. Pharm Res. 2012;29(2):345–51.
Gao Z. In vitro dissolution testing with flow-through method: a technical note. AAPS PharmSciTech. 2009;10(4):1401–5.
Baxter JL, Kukura J, Muzzio FJ. Hydrodynamics-induced variability in the USP apparatus II dissolution test. Int J Pharm. 2005;292(1–2):17–28.
Cammarn SR, Sakr A. Predicting dissolution via hydrodynamics: salicylic acid tablets in flow through cell dissolution. Int J Pharm. 2000;201(2):199–209.
D’Arcy DM, Liu B, Bradley G, Healy AM, Corrigan OI. Hydrodynamic and species transfer simulations in the USP 4 dissolution apparatus: considerations for dissolution in a low velocity pulsing flow. Pharm Res. 2010;27(2):246–58.
D’Arcy DM, Liu B, Corrigan OI. Investigating the effect of solubility and density gradients on local hydrodynamics and drug dissolution in the USP 4 dissolution apparatus. Int J Pharm. 2011;419(1–2):175–85.
Kukura J, Baxter JL, Muzzio FJ. Shear distribution and variability in the USP apparatus 2 under turbulent conditions. Int J Pharm. 2004;279(1–2):9–17.
Morihara M, Aoyagi N, Kaniwa N, Katori N, Kojim S. Hydrodynamic flows around tablets in different pharmacopeial dissolution tests. Drug Dev Ind Pharm. 2002;28(6):655–62.
Kakhi M. Classification of the flow regimes in the flow-through cell. Eur J Pharm Sci. 2009;37(5):531–44.
Shiko G, Sederman AJ, Gladden LF. MRI technique for the snapshot imaging of quantitative velocity maps using RARE. J Magn Reson. 2012;216:183–91.
Yoshida H, Kuwana A, Shibata H, Izutsu K, Goda Y. Particle image velocimetry evaluation of fluid flow profiles in USP 4 flow-through dissolution cells. Pharm Res. 2015;32(9):2950–9.
Krämer J, Stippler E. Experiences with USP apparatus 4 calibration. Dissolution Technol. 2005;12(2):33–9.
FDA Guidance for Industry Dissolution Testing of Immediate. Release Solid Oral Dosage Forms U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), August 1997.
Brown W. Apparatus 4 flow through cell: some thoughts on operational characteristics. Dissolution Technol. 2005;12(2):28–30.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors would like to thank Mr. Shimizu and Mr. Nakatani (Dainippon Seiki Co., Ltd.) for making the DF-7 FTC system available. This work is partly supported by the Research on Regulatory Harmonization and Evaluation of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics from the Japan Agency for Medical Research and Development (AMED), and by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour, and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, H., Kuwana, A., Shibata, H. et al. Effects of Pump Pulsation on Hydrodynamic Properties and Dissolution Profiles in Flow-Through Dissolution Systems (USP 4). Pharm Res 33, 1327–1336 (2016). https://doi.org/10.1007/s11095-016-1874-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1874-8