Abstract
Purpose
Aggregation aspects of therapeutic monoclonal antibodies (mAbs) are of common concern to the pharmaceutical industry. Low pH treatment is applied during affinity purification and to inactivate endogenous retroviruses, directing interest to the mechanisms of acid-induced antibody aggregation.
Methods
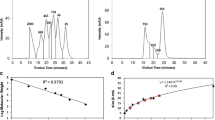
We characterized the oligomerization kinetics at pH 3.3, as well as the reversibility upon neutralization, of three model mAbs with identical variable regions, representative of IgG1, IgG2 and IgG4 respectively. We applied size-exclusion high performance liquid chromatography and orthogonal analytical methods, including small-angle X-ray scattering and dynamic light scattering and supplemented the experimental data with crystal structure-based spatial aggregation propensity (SAP) calculations.
Results
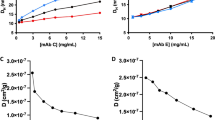
We revealed distinct solution behaviors between the three mAb models: At acidic pH IgG1 retained monomeric, whereas IgG2 and IgG4 exhibited two-phase oligomerization processes. After neutralization, IgG2 oligomers partially reverted to the monomeric state, while on the contrary, IgG4 oligomers tended to aggregate. Subclass-specific aggregation-prone motifs on the Fc fragments were identified, which may lead to two distinct pathways of reversible and irreversible aggregation, respectively.
Conclusions
We conclude that subtle variations in mAb sequence greatly affect responses towards low-pH incubation and subsequent neutralization, and demonstrate how orthogonal biophysical methods distinguish between reversible and irreversible mAb aggregation pathways at early stages of acidic treatment.








Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- AUP:
-
Area under the peak
- DLS:
-
Dynamic light scattering
- Fab:
-
Antigen-binding fragment
- Fc:
-
Crystallizable fragment
- HMWS:
-
High molecular weight species
- HPLC:
-
High-performance liquid chromatography
- I0 :
-
Forward scattering intensity
- Ig:
-
Immunoglobulin
- mAb:
-
Monoclonal antibody
- MALS:
-
Multi-angle static light scattering
- MW:
-
Molecular weight
- P(r):
-
Pair distance distribution function
- PBS:
-
Phosphate buffered saline
- PDB:
-
Protein data bank
- pI:
-
Isoelectric point
- q :
-
Length of the scattering vector
- Rg :
-
Radius of gyration
- Rh :
-
Hydrodynamic radius
- SAP:
-
Spatial aggregation propensity
- SASA:
-
Solvent accessible surface area
- SAXS:
-
Small-angle X-ray scattering
- SEC:
-
Size-exclusion chromatography
- Tm :
-
Melting point
- UV:
-
Ultraviolet
References
Aggarwal RS. What’s fueling the biotech engine-2012 to 2013. Nat Biotechnol. 2014;32(1):32–9.
Poiron C, Wu Y, Ginestoux C, Ehrenmann F, Duroux P, Lefranc M. IMGT/mAb-DB: the IMGT® database for therapeutic monoclonal antibodies. Poster no101. 2010;11.
Krishnan S, Pallitto MM, Ricci MS. Development of formulations for therapeutic monoclonal antibodies and Fc fusion proteins. Formulation and Process Development Strategies for Manufacturing Biopharmaceuticals. 2010. p. 383–427.
Potty ASP, Xenopoulos A. Stress-induced antibody aggregates. Bioproc Int. 2013;11(3):44–52.
Andersen CB, Manno M, Rischel C, Thorolfsson M, Martorana V. Aggregation of a multidomain protein: a coagulation mechanism governs aggregation of a model IgG1 antibody under weak thermal stress. Protein Sci. 2010;19(2):279–90.
Arosio P, Rima S, Morbidelli M. Aggregation mechanism of an IgG2 and two IgG1 monoclonal antibodies at low pH: from oligomers to larger aggregates. Pharm Res. 2013;30(3):641–54.
Tian X, Langkilde AE, Thorolfsson M, Rasmussen HB, Vestergaard B. Small-angle X-ray scattering screening complements conventional biophysical analysis: comparative structural and biophysical analysis of monoclonal antibodies IgG1, IgG2, and IgG4. J Pharm Sci. 2014;103(6):1701–10.
Ito T, Tsumoto K. Effects of subclass change on the structural stability of chimeric, humanized, and human antibodies under thermal stress. Protein Sci. 2013;22(11):1542–51.
Ishikawa T, Ito T, Endo R, Nakagawa K, Sawa E, Wakamatsu K. Influence of pH on heat-induced aggregation and degradation of therapeutic monoclonal antibodies. Biol Pharm Bull. 2010;33(8):1413–7.
Latypov RF, Hogan S, Lau H, Gadgil H, Liu DJ. Elucidation of acid-induced unfolding and aggregation of human immunoglobulin IgG1 and IgG2 Fc. J Biol Chem. 2012;287(2):1381–96.
Garber E, Demarest SJ. A broad range of Fab stabilities within a host of therapeutic IgGs. Biochem Biophys Res Commun. 2007;355(3):751–7.
Chennamsetty N, Helk B, Voynov V, Kayser V, Trout BL. Aggregation-prone motifs in human immunoglobulin G. J Mol Biol. 2009;391(2):404–13.
Chennamsetty N, Voynov V, Kayser V, Helk B, Trout BL. Design of therapeutic proteins with enhanced stability. Proc Natl Acad Sci U S A. 2009;106(29):11937–42.
Blanchet CE, Zozulya AV, Kikhney AG, Franke D, Konarev PV, Shang W, et al. Instrumental setup for high-throughput small- and wide-angle solution scattering at the X33 beamline of EMBL Hamburg. J Appl Crystallogr. 2012;45(3):489–95.
Petoukhov MV, Franke D, Shkumatov AV, Tria G, Kikhney AG, Gajda M, et al. New developments in the ATSAS program package for small-angle scattering data analysis. J Appl Crystallogr. 2012;45:342–50.
Svergun DI. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J Appl Crystallogr. 1992;25:495–503.
Molecular Operating Environment (MOE), 2013.08; Chemical Computing Group Inc., 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2014.
Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32:W665-7.
Karkov HS, Krogh BO, Woo J, Parimal S, Ahmadian H, Cramer SM. Investigation of protein selectivity in multimodal chromatography using in silico designed Fab fragment variants. Biotechnol Bioeng. 2015.
Tian X, Vestergaard B, Thorolfsson M, Yang Z, Rasmussen HB, Langkilde AE. In-depth analysis of subclass-specific conformational preferences of IgG antibodies. IUCrJ. 2015;2. doi: 10.1107/S205225251402209X.
Mosbaek CR, Konarev PV, Svergun DI, Rischel C, Vestergaard B. High concentration formulation studies of an IgG2 antibody using small angle X-ray scattering. Pharm Res. 2012;29(8):2225–35.
Eryilmaz E, Janda A, Kim J, Cordero RJ, Cowburn D, Casadevall A. Global structures of IgG isotypes expressing identical variable regions. Mol Immunol. 2013;56(4):588–98.
Lilyestrom WG, Yadav S, Shire SJ, Scherer TM. Monoclonal antibody self-association, cluster formation, and rheology at high concentrations. J Phys Chem B. 2013;117(21):6373–84.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors thank Zhiru Yang for her support with protein production, Mikkel Melchior Rasmussen for his assistance with biophysical analyses. We are grateful for the availability of beamtime and we thank the beamline staff for their great help at P12 EMBL/PETRA III, Hamburg. Funding from the Drug Research Academy, Novo Nordisk A/S, Carlsberg Foundation, Danish Council for Independent Research, Sapere Aude programme and DANSCATT is sincerely acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Thomas Skamris and Xinsheng Tian contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 43509 kb)
Rights and permissions
About this article
Cite this article
Skamris, T., Tian, X., Thorolfsson, M. et al. Monoclonal Antibodies Follow Distinct Aggregation Pathways During Production-Relevant Acidic Incubation and Neutralization. Pharm Res 33, 716–728 (2016). https://doi.org/10.1007/s11095-015-1821-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1821-0