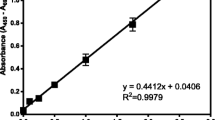
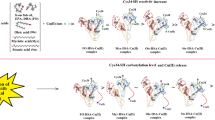
ABSTRACT
Purpose
Ascorbic acid has been considered as a potential radical scavenging excipient for pharmaceutical formulations. However, under certain circumstances, ascorbic acid can generate reactive oxygen species via redox cycling. The objective of this study was to investigate ascorbic acid-induced oxidative carbonylation of therapeutic proteins and correlate the increase in carbonylation with protein aggregation.
Methods
An optimized ELISA for quantifying carbonyl levels was used to compare the oxidizing potentials of ascorbic acid and hydrogen peroxide by testing four pharmaceutically-relevant proteins (human serum albumin, immunoglobulin G, granulocyte-colony stimulating factor and calcitonin). Several transition metals at micromolar concentrations were evaluated for their ability to enhance ascorbic acid-induced protein carbonylation. Protein aggregation under oxidative conditions, with or without free radical scavengers, was measured by aggregate binding fluorescent dye and confirmed by microfluidic imaging.
Results
Addition of ascorbic acid alone resulted in higher increases in carbonylation than addition of hydrogen peroxide. The presence of trace amounts (>75 ppb) of copper enhanced oxidative effects of ascorbic acid, whereas other tested metals did not comparably promote oxidation. During oxidation, protein destabilization indicated by loss of the full-length protein, positively correlated with the increase in protein aggregation. However, levels of aggregation did not always correlate with the levels of protein carbonylation. At comparable carbonylation levels, addition of copper produced greater protein destabilization and aggregation than addition of iron.
Conclusions
The results strongly suggest that ascorbic acid with traces of metals, especially copper, can promote therapeutic protein carbonylation and potentially aggregation. At similar carbonylation levels, some oxidative conditions may lead to greater protein destabilization than others.







Similar content being viewed by others
Abbreviations
- ATP:
-
Adenosine triphosphate
- DCVJ:
-
9-(dicyanovinyl)julolidine
- DNP:
-
2,4-dinitrophenyl hydrazone
- DNPH:
-
2,4-dinitrophenyl hydrazine
- ELISA:
-
Enzyme-linked immunosorbent assay
- G-CSF:
-
Granulocyte-colony stimulating factor
- HSA:
-
Recombinant human serum albumin
- IgG:
-
Immunoglobulin G
- MFI:
-
Microfluidic imaging
- ROS:
-
Reactive oxygen species
REFERENCES
Ott C, Grune T. Protein oxidation and proteolytic signalling in aging. Curr Pharm Des. 2014;20(18):3040–51.
Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, et al. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. 2015;16(1):193–217.
Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95.
Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metab Rev. 2000;32(3–4):307–26.
Stadtman ER, Berlett BS. Fenton chemistry. Amino acid oxidation. J Biol Chem. 1991;266(26):17201–11.
Torosantucci R, Schoneich C, Jiskoot W. Oxidation of therapeutic proteins and peptides: structural and biological consequences. Pharm Res. 2014;31(3):541–53.
Yang Y, Stella C, Wang W, Schoneich C, Gennaro L. Characterization of oxidative carbonylation on recombinant monoclonal antibodies. Anal Chem. 2014;86(10):4799–806.
Madian AG, Regnier FE. Proteomic identification of carbonylated proteins and their oxidation sites. J Proteome Res. 2010;9(8):3766–80.
Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med. 2006;10(2):389–406.
Bollineni RC, Fedorova M, Bluher M, Hoffmann R. Carbonylated plasma proteins as potential biomarkers of obesity induced type 2 diabetes mellitus. J Proteome Res. 2014;13(11):5081–93.
Randolph TW, Schiltz E, Sederstrom D, Steinmann D, Mozziconacci O, Schoneich C, et al. Do not drop: mechanical shock in vials causes cavitation, protein aggregation, and particle formation. J Pharm Sci. 2015;104(2):602–11.
Uehara H, Rao VA. Metal-mediated protein oxidation: applications of a modified ELISA-based carbonyl detection assay for complex proteins. Pharm Res.2015;32(2):691–701.
Joubert MK, Luo Q, Nashed-Samuel Y, Wypych J, Narhi LO. Classification and characterization of therapeutic antibody aggregates. J Biol Chem. 2011;286(28):25118–33.
Wang W, Ignatius AA, Thakkar SV. Impact of residual impurities and contaminants on protein stability. J Pharm Sci. 2014;103(5):1315–30.
Seidl A, Hainzl O, Richter M, Fischer R, Bohm S, Deutel B, et al. Tungsten-induced denaturation and aggregation of epoetin alfa during primary packaging as a cause of immunogenicity. Pharm Res. 2012;29(6):1454–67.
Gao X, Ji JA, Veeravalli K, Wang YJ, Zhang T, McGreevy W, et al. Effect of individual Fc methionine oxidation on FcRn binding: Met252 oxidation impairs FcRn binding more profoundly than Met428 oxidation. J Pharm Sci. 2015;104(2):368–77.
Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8(3):E501–7.
Ratanji KD, Derrick JP, Dearman RJ, Kimber I. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol. 2014;11(2):99–109.
Chang BS, Hershenson S. Practical approaches to protein formulation development. Pharm Biotechnol. 2002;13:1–25.
Jorgensen L, Hostrup S, Moeller EH, Grohganz H. Recent trends in stabilising peptides and proteins in pharmaceutical formulation—considerations in the choice of excipients. Expert Opin Drug Deliv. 2009;6(11):1219–30.
Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996;145(5):532–41.
Zbikowska HM, Nowak P, Wachowicz B. Protein modification caused by a high dose of gamma irradiation in cryo-sterilized plasma: protective effects of ascorbate. Free Radic Biol Med. 2006;40(3):536–42.
Kerwin BA, Heller MC, Levin SH, Randolph TW. Effects of Tween 80 and sucrose on acute short-term stability and long-term storage at −20 degrees C of a recombinant hemoglobin. J Pharm Sci. 1998;87(9):1062–8.
Cheng KT. 131I-Tositumomab. In: Molecular Imaging and Contrast Agent Database (MICAD). [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. Available from: http://www.ncbi.nlm.nih.gov/books/NBK23253/
de Blois E, Chan HS, de Zanger R, Konijnenberg M, Breeman WA. Application of single-vial ready-for-use formulation of 111In- or 177Lu-labelled somatostatin analogs. Appl Radiat Isot. 2014;85:28–33.
Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826(2):443–57.
Bollineni RC, Fedorova M, Hoffmann R. Qualitative and quantitative evaluation of derivatization reagents for different types of protein-bound carbonyl groups. Analyst. 2013;138(17):5081–8.
Aryal B, Jeong J, Rao VA. Doxorubicin-induced carbonylation and degradation of cardiac myosin binding protein C promote cardiotoxicity. Proc Natl Acad Sci U S A. 2014;111(5):2011–6.
Dahms SO, Konnig I, Roeser D, Guhrs KH, Mayer MC, Kaden D, et al. Metal binding dictates conformation and function of the amyloid precursor protein (APP) E2 domain. J Mol Biol. 2012;416(3):438–52.
Herman AC, Boone TC, Lu HS. Characterization, formulation, and stability of Neupogen (Filgrastim), a recombinant human granulocyte-colony stimulating factor. Pharm Biotechnol. 1996;9:303–28.
Capelle MA, Gurny R, Arvinte T. A high throughput protein formulation platform: case study of salmon calcitonin. Pharm Res. 2009;26(1):118–28.
Neergaard MS, Nielsen AD, Parshad H, Van De Weert M. Stability of monoclonal antibodies at high-concentration: head-to-head comparison of the IgG1 and IgG4 subclass. J Pharm Sci. 2014;103(1):115–27.
Fliszar KA, Walker D, Allain L. Profiling of metal ions leached from pharmaceutical packaging materials. PDA J Pharm Sci Technol. 2006;60(6):337–42.
Shen D, Coleman J, Chan E, Nicholson TP, Dai L, Sheppard PW, et al. Novel cell- and tissue-based assays for detecting misfolded and aggregated protein accumulation within aggresomes and inclusion bodies. Cell Biochem Biophys. 2011;60(3):173–85.
Hawe A, Filipe V, Jiskoot W. Fluorescent molecular rotors as dyes to characterize polysorbate-containing IgG formulations. Pharm Res. 2010;27(2):314–26.
Abdallah DM, El-Abhar HS, Abdel-Aziz DH. TEMPOL, a membrane-permeable radical scavenger, attenuates gastric mucosal damage induced by ischemia/reperfusion: a key role for superoxide anion. Eur J Pharmacol. 2009;603(1–3):93–7.
Rahhal S, Richter HW. Reaction of hydroxyl radicals with the ferrous and ferric iron chelates of diethylenetriamine-N, N, N', N", N"- pentaacetate. Free Radic Res Commun. 1989;6(6):369–77.
Knepp VM, Whatley JL, Muchnik A, Calderwood TS. Identification of antioxidants for prevention of peroxide-mediated oxidation of recombinant human ciliary neurotrophic factor and recombinant human nerve growth factor. PDA J Pharm Sci Technol. 1996;50(3):163–71.
Blessy MRDP, Prajapati PN, Agrawal YK. Development of forced degradation and stability indicating studies of drugs—a review. J Pharm Anal. 2014;4(3):159–65.
Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272(33):20313–6.
Stadtman ER. Ascorbic acid and oxidative inactivation of proteins. Am J Clin Nutr. 1991;54(6 Suppl):1125S–8S.
Parenky A, Myler H, Amaravadi L, Bechtold-Peters K, Rosenberg A, Kirshner S, et al. New FDA draft guidance on immunogenicity. AAPS J. 2014;16(3):499–503.
van Beers MM, Sauerborn M, Gilli F, Brinks V, Schellekens H, Jiskoot W. Oxidized and aggregated recombinant human interferon beta is immunogenic in human interferon beta transgenic mice. Pharm Res. 2011;28(10):2393–402.
Carty JL, Bevan R, Waller H, Mistry N, Cooke M, Lunec J, et al. The effects of vitamin C supplementation on protein oxidation in healthy volunteers. Biochem Biophys Res Commun. 2000;273(2):729–35.
Desai V, Kaler SG. Role of copper in human neurological disorders. Am J Clin Nutr. 2008;88(3):855S–8S.
Teitzel GM, Geddie A, De Long SK, Kirisits MJ, Whiteley M, Parsek MR. Survival and growth in the presence of elevated copper: transcriptional profiling of copper-stressed Pseudomonas aeruginosa. J Bacteriol. 2006;188(20):7242–56.
Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821.
Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25(3–4):207–18.
Steinmann D, Ji JA, Wang YJ, Schoneich C. Oxidation of human growth hormone by oxygen-centered radicals: formation of Leu-101 hydroperoxide and Tyr-103 oxidation products. Mol Pharm. 2012;9(4):803–14.
Bee JS, Davis M, Freund E, Carpenter JF, Randolph TW. Aggregation of a monoclonal antibody induced by adsorption to stainless steel. Biotechnol Bioeng. 2010;105(1):121–9.
Urbanski NK, Beresewicz A. Generation of *OH initiated by interaction of Fe2+ and Cu+ with dioxygen; comparison with the Fenton chemistry. Acta Biochim Pol. 2000;47(4):951–62.
Kroneck PM, Vortisch V, Hemmerich P. Model studies on the coordination of copper in biological systems. The deprotonated peptide nitrogen as a potential binding site for copper(II). Eur J Biochem. 1980;109(2):603–12.
ACKNOWLEDGMENTS AND DISCLOSURES
This research was supported by the CDER Critical Path Initiative. We thank Dr. Shen Luo for help with microfluidic imaging. We would like to thank Dr. Hiroshi Uehara and Elliot Rosen (FDA) for critical reading of the manuscript. The authors have no competing financial interests to disclose. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Food and Drug Administration and the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Evaluation of oxidative effects of ascorbic acid in the presence of metal chelator EDTA. Each of the three proteins (HSA, IgG and G-CSF) was incubated for 16 h at 25°C alone or with addition of the indicated compounds (5 mM ascorbic acid or 5 mM ascorbic acid with 3 mM EDTA). After oxidation proteins were derivatized with DNPH and analyzed by (A) carbonyl immunoblotting with antibodies against DNP group, (B) carbonyl ELISA to quantify protein carbonylation. Carbonyl values were normalized to the control reactions (protein only) and shown as carbonylation fold increases. Data are shown as mean ± SD. * p < 0.05 (GIF 21 kb)
Rights and permissions
About this article
Cite this article
Kryndushkin, D., Rao, V.A. Comparative Effects of Metal-Catalyzed Oxidizing Systems on Carbonylation and Integrity of Therapeutic Proteins. Pharm Res 33, 526–539 (2016). https://doi.org/10.1007/s11095-015-1807-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1807-y