ABSTRACT
Purpose
Increased solution viscosity results in difficulties in manufacturing and delivery of therapeutic protein formulations, increasing both the time and production costs, and leading to patient inconvenience. The solution viscosity is affected by the molecular properties of both the solute and the solvent. The purpose of this work was to investigate the effect of size, charge and protein-protein interactions on the viscosity of Dual Variable Domain Immunoglobulin (DVD-IgTM) protein solutions.
Methods
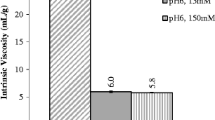
The effect of size of the protein molecule on solution viscosity was investigated by measuring intrinsic viscosity and excluded volume calculations for monoclonal antibody (mAb) and DVD-IgTM protein solutions. The role of the electrostatic charge resulting in electroviscous effects for DVD-IgTM protein was assessed by measuring zeta potential. Light scattering measurements were performed to detect protein-protein interactions affecting solution viscosity.
Results
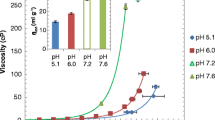
DVD-IgTM protein exhibited significantly higher viscosity compared to mAb. Intrinsic viscosity and excluded volume calculations indicated that the size of the molecule affects viscosity significantly at higher concentrations, while the effect was minimal at intermediate concentrations. Electroviscous contribution to the viscosity of DVD-IgTM protein varied depending on the presence or absence of ions in the solution. In buffered solutions, negative k D and B 2 values indicated the presence of attractive interactions which resulted in high viscosity for DVD-IgTM protein at certain pH and ionic strength conditions.
Conclusions
Results show that more than one factor contributes to the increased viscosity of DVD-IgTM protein and interplay of these factors modulates the overall viscosity behavior of the solution, especially at higher concentrations.










Similar content being viewed by others
Abbreviations
- DLS:
-
Dynamic light scattering
- DVD-IgTM protein:
-
Dual variable domain immunoglobulin protein
- LLPS:
-
Liquid-liquid phase separation
- mAb:
-
Monoclonal antibody
- PPI:
-
Protein-protein Interactions
- SC:
-
Subcutaneous
- SLS:
-
Static light scattering
REFERENCES
Evans JB, Syed BA. From the analyst’s couch: next-generation antibodies. Nat Rev Drug Discov. 2014;13(6):413–4.
Reichert JM, Beck A, Lugovskoy AA, Wurch T, Coats S, Brezski RJ. 9th annual European antibody congress, November 11-13, 2013, Geneva, Switzerland. MAbs. 2014;6(2):309–26.
Harris RJSS, Winter C. Commercial manufacturing scale formulation and analytical characterization of therapeutic recombinant antibodies. Drug Dev Res. 2004;61:137–54.
Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93(6):1390–402.
Daugherty AL, Mrsny RJ. Formulation and delivery issues for monoclonal antibody therapeutics. Adv Drug Deliv Rev. 2006;58(5–6):686–706.
Burckbuchler V, Mekhloufi G, Giteau AP, Grossiord JL, Huille S, Agnely F. Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions. Eur J Pharm Biopharm. 2010;76(3):351–6.
Allmendinger A, Fischer S, Huwyler J, Mahler H-C, Schwarb E, Zarraga IE, et al. Rheological characterization and injection forces of concentrated protein formulations: An alternative predictive model for non-Newtonian solutions. Eur J Pharm Biopharm. 2014;87(2):318–28.
Simha R. Effect of concentration on the viscosity of dilute solutions. J Res Natl Bur Stand. 1949;42:409–17.
Hall D, Minton AP. Macromolecular crowding: qualitative and semiquantitative successes, quantitative challenges. Biochim Biophys Acta. 2003;1649(2):127–39.
Zhou HX, Rivas G, Minton AP. Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys. 2008;37:375–97.
Mooney M. The viscosity of a concentrated suspension of spherical particles. J Colloid Sci. 1951;6(2):162–70.
Ross PD, Minton AP. Hard quasispherical model for the viscosity of hemoglobin solutions. Biochem Biophys Res Commun. 1977;76(4):971–6.
Liu J, Nguyen MD, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci. 2005;94(9):1928–40.
Kanai S, Liu J, Patapoff TW, Shire SJ. Reversible self-association of a concentrated monoclonal antibody solution mediated by Fab-Fab interaction that impacts solution viscosity. J Pharm Sci. 2008;97(10):4219–27.
Yadav S, Liu J, Shire SJ, Kalonia DS. Specific interactions in high concentration antibody solutions resulting in high viscosity. J Pharm Sci. 2010;99(3):1152–68.
Yadav S, Shire SJ, Kalonia DS. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J Pharm Sci. 2010;99(12):4812–29.
Yadav S, Laue TM, Kalonia DS, Singh SN, Shire SJ. The influence of charge distribution on self-association and viscosity behavior of monoclonal antibody solutions. Mol Pharm. 2012;9(4):791–802.
Connolly BD, Petry C, Yadav S, Demeule B, Ciaccio N, Moore JM, et al. Weak interactions govern the viscosity of concentrated antibody solutions: high-throughput analysis using the diffusion interaction parameter. Biophys J. 2012;103(1):69–78.
Zarraga IE, Taing R, Zarzar J, Luoma J, Hsiung J, Patel A, et al. High shear rheology and anisotropy in concentrated solutions of monoclonal antibodies. J Pharm Sci. 2013;102(8):2538–49.
Yearley Eric J, Zarraga Isidro E, Shire Steven J, Scherer Thomas M, Gokarn Y, Wagner Norman J, et al. Small-angle neutron scattering characterization of monoclonal antibody conformations and interactions at high concentrations. Biophys J. 2013;105(3):720–31.
Yadav S, Shire SJ, Kalonia DS. Viscosity behavior of high-concentration monoclonal antibody solutions: correlation with interaction parameter and electroviscous effects. J Pharm Sci. 2012;101(3):998–1011.
Salinas BA, Sathish HA, Bishop SM, Harn N, Carpenter JF, Randolph TW. Understanding and modulating opalescence and viscosity in a monoclonal antibody formulation. J Pharm Sci. 2010;99(1):82–93.
Yearley EJ, Godfrin PD, Perevozchikova T, Zhang H, Falus P, Porcar L, et al. Observation of small cluster formation in concentrated monoclonal antibody solutions and its implications to solution viscosity. Biophys J. 2014;106(8):1763–70.
Du W, Klibanov AM. Hydrophobic salts markedly diminish viscosity of concentrated protein solutions. Biotechnol Bioeng. 2011;108(3):632–6.
Guo Z, Chen A, Nassar RA, Helk B, Mueller C, Tang Y, et al. Structure-activity relationship for hydrophobic salts as viscosity-lowering excipients for concentrated solutions of monoclonal antibodies. Pharm Res. 2012;29(11):3102–9.
Kamerzell TJ, Pace AL, Li M, Danilenko DM, McDowell M, Gokarn YR, et al. Polar solvents decrease the viscosity of high concentration IgG1 solutions through hydrophobic solvation and interaction: formulation and biocompatibility considerations. J Pharm Sci. 2013;102(4):1182–93.
He F, Woods CE, Trilisky E, Bower KM, Litowski JR, Kerwin BA, et al. Screening of monoclonal antibody formulations based on high-throughput thermostability and viscosity measurements: design of experiment and statistical analysis. J Pharm Sci. 2011;100(4):1330–40.
Inoue N, Takai E, Arakawa T, Shiraki K. Specific decrease in solution viscosity of antibodies by arginine for therapeutic formulations. Mol Pharm. 2014;11(6):1889–96.
Chen B, Bautista R, Yu K, Zapata G, Mulkerrin M, Chamow S. Influence of histidine on the stability and physical properties of a fully human antibody in aqueous and solid forms. Pharm Res. 2003;20(12):1952–60. English.
Yadav S, Shire SJ, Kalonia DS. Viscosity analysis of high concentration bovine serum albumin aqueous solutions. Pharm Res. 2011;28(8):1973–83.
Schmit JD, He F, Mishra S, Ketchem RR, Woods CE, Kerwin BA. Entanglement model of antibody viscosity. J Phys Chem B. 2014;118(19):5044–9.
Sarangapani Prasad S, Hudson Steven D, Migler Kalman B, Pathak Jai A. The limitations of an exclusively colloidal view of protein solution hydrodynamics and rheology. Biophys J. 2013;105(10):2418–26.
Hunter RJ. Zeta potential in colloid science: principles and applications. London: Academic; 1981.
Raut AS, Kalonia DS. Opalescence in monoclonal antibody solutions and its correlation with intermolecular interactions in dilute and concentrated solutions. J Pharm Sci. 2015;104(4):1263–74.
Harding SE, Johnson P. The concentration-dependence of macromolecular parameters. Biochem J. 1985;231(3):543–47.
Berne BJ, Pecora R. Dynamic light scattering: with applications to chemistry, biology, and physics. Mineola: Courier Dover Publications; 2000.
George A, Wilson WW. Predicting protein crystallization from a dilute solution property. Acta Crystallogr D Biol Crystallogr. 1994;50(Pt 4):361–5.
Yadav S, Scherer TM, Shire SJ, Kalonia DS. Use of dynamic light scattering to determine second virial coefficient in a semidilute concentration regime. Anal Biochem. 2011;411(2):292–6.
Hiemenz PC, Rajagopalan R. Principles of colloid and surface chemistry, revised and expanded. New York: CRC Press; 1997.
Saito S, Hasegawa J, Kobayashi N, Kishi N, Uchiyama S, Fukui K. Behavior of monoclonal antibodies: relation between the second virial coefficient (B (2)) at low concentrations and aggregation propensity and viscosity at high concentrations. Pharm Res. 2012;29(2):397–410.
Chari R, Jerath K, Badkar AV, Kalonia DS. Long- and short-range electrostatic interactions affect the rheology of highly concentrated antibody solutions. Pharm Res. 2009;26(12):2607–18.
Saluja A, Badkar AV, Zeng DL, Nema S, Kalonia DS. Ultrasonic storage modulus as a novel parameter for analyzing protein-protein interactions in high protein concentration solutions: correlation with static and dynamic light scattering measurements. Biophys J. 2007;92(1):234–44.
Harding SE. The intrinsic viscosity of biological macromolecules. Progress in measurement, interpretation and application to structure in dilute solution. Prog Biophys Mol Biol. 1997;68(2–3):207–62.
Mehl JW, Oncley JL, Simha R. Viscosity and the shape of protein molecules. Science. 1940;92(2380):132–3.
Solomon OF, Ciutǎ IZ. Détermination de la viscosité intrinsèque de solutions de polymères par une simple détermination de la viscosité. J Appl Polym Sci. 1962;6(24):683–6.
Saluja A, Kalonia DS. Application of ultrasonic shear rheometer to characterize rheological properties of high protein concentration solutions at microliter volume. J Pharm Sci. 2005;94(6):1161–8.
Neal BL, Lenhoff AM. Excluded volume contribution to the osmotic second virial coefficient for proteins. AICHE J. 1995;41(4):1010–4.
Lilyestrom WG, Yadav S, Shire SJ, Scherer TM. Monoclonal antibody self-association, cluster formation, and rheology at high concentrations. J Phys Chem B. 2013;117(21):6373–84.
Zholkovskiy EK, Adeyinka OB, Masliyah JH. Spherical cell approach for the effective viscosity of suspensions. J Phys Chem B. 2006;110(39):19726–34.
Laue T. Proximity energies: a framework for understanding concentrated solutions. J Mol Recognit: JMR. 2012;25(3):165–73.
Israelachvili JN. Intermolecular and surface forces. 3rd ed. San Diego: Academic; 2011.
Bull HB. The electroviscous effect in egg albumin solutions. Trans Faraday Soc. 1940;35:80–4.
Buzzell JG, Tanford C. The effect of charge and ionic strength on the viscosity of ribonuclease. J Phys Chem. 1956;60(9):1204–7.
Tanford C, Buzzell JG. The viscosity of aqueous solutions of bovine serum albumin between pH 4.3 and 10.5. J Phys Chem. 1956;60(2):225–31.
ACKNOWLEDGMENTS AND DISCLOSURES
Authors would like to thank AbbVie Bioresearch Center, Worcester, MA for material and financial support for the work.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
A brief description of T cloud as important parameters to measure PPI in solution is provided in the supporting material. Plot of T cloud for DVD-IgTM as a function of solution conditions is also elaborated. Excluded volume effect using modified Ross and Minton equation for mAb and DVD-IgTM is briefly discussed in the Supporting Information. (DOCX 146 kb)
Rights and permissions
About this article
Cite this article
Raut, A.S., Kalonia, D.S. Viscosity Analysis of Dual Variable Domain Immunoglobulin Protein Solutions: Role of Size, Electroviscous Effect and Protein-Protein Interactions. Pharm Res 33, 155–166 (2016). https://doi.org/10.1007/s11095-015-1772-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1772-5