Abstract
Purpose
The objective of this study was to compare two different nebulizers: Eflow rapid® and Pari LC star® by scintigraphy and PK modeling to simulate epithelial lining fluid concentrations from measured plasma concentrations, after nebulization of CMS in baboons.
Methods
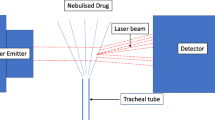
Three baboons received CMS by IV infusion and by 2 types of aerosols generators and colistin by subcutaneous infusion. Gamma imaging was performed after nebulisation to determine colistin distribution in lungs. Blood samples were collected during 9 h and colistin and CMS plasma concentrations were measured by LC-MS/MS. A population pharmacokinetic analysis was conducted and simulations were performed to predict lung concentrations after nebulization.
Results
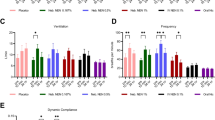
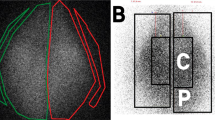
Higher aerosol distribution into lungs was observed by scintigraphy, when CMS was nebulized with Pari LC® star than with Eflow Rapid® nebulizer. This observation was confirmed by the fraction of CMS deposited into the lung (respectively 3.5% versus 1.3%).CMS and colistin simulated concentrations in epithelial lining fluid were higher after using the Pari LC star® than the Eflow rapid® system.
Conclusions
A limited fraction of CMS reaches lungs after nebulization, but higher colistin plasma concentrations were measured and higher intrapulmonary colistin concentrations were simulated with the Pari LC Star® than with the Eflow Rapid® system.





Similar content being viewed by others
Abbreviations
- 99m Tc-DTPA:
-
99m technetium-diethylene triamino pentaacetic acid
- AUCELF :
-
Area under the ELF concentrations-time curve
- BAL:
-
Broncho-alveolar lavage
- C:
-
Central lung
- CBA:
-
Colistin base activity
- CF:
-
Cystic fibrosis
- CMS:
-
Colistin methansulphonate
- ELF:
-
Epithelial lining fluid
- ET:
-
Extrathoracic
- GDS:
-
Geometric standard deviation
- HPLC:
-
High-performance liquid chromatography
- IIV:
-
Inter-individual variability
- IM:
-
Intramuscular
- IOV:
-
Inter-occasion variability
- IV:
-
Intravenous
- LC-MS/MS:
-
Liquid chromatography coupled with tandem mass spectrometry
- LLOQ:
-
Lower limit of quantification
- MMAD:
-
Mass median aerodynamic diameter
- NLME:
-
Non-linear mixed effects
- OFV:
-
Objective function value
- P:
-
Peripheral lung
- PK:
-
Pharmacokinetics
- ROIs:
-
Regions of interest
- SC:
-
Subcutaneous
- T:
-
Lung
- TH:
-
Thoracic
- VAP:
-
Ventilator-associated pneumonia
- VPC:
-
Visual predictive checks
References
Li J, Coulthard K, Milne R, Nation RL, Conway S, Peckham D, et al. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother. 2003;52(6):987–92.
Marchetti F, Giglio L, Candusso M, Faraguna D, Assael BM. Early antibiotic treatment of pseudomonas aeruginosa colonisation in cystic fibrosis: a critical review of the literature. Eur J Clin Pharmacol. 2004;60(2):67–74.
Berlana D, Llop JM, Fort E, Badia MB, Jodar R. Use of colistin in the treatment of multiple-drug-resistant gram-negative infections. Am J Health Syst Pharm. 2005;62(1):39–47.
Falagas ME, Kasiakou SK, Tsiodras S, Michalopoulos A. The use of intravenous and aerosolized polymyxins for the treatment of infections in critically ill patients: a review of the recent literature. Clin Med Res. 2006;4(2):138–46.
Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6(9):589–601.
Michalopoulos A, Kasiakou SK, Mastora Z, Rellos K, Kapaskelis AM, Falagas ME. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Crit Care. 2005;9(1):R53–59.
Korbila IP, Michalopoulos A, Rafailidis PI, Nikita D, Samonis G, Falagas ME. Inhaled colistin as adjunctive to intravenous colistin for the treatment of microbiologically documented VAP: a comparative cohort study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2009.
Michalopoulos A, Fotakis D, Virtzili S, Vletsas C, Raftopoulou S, Mastora Z, et al. Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant gram-negative bacteria: a prospective study. Respir Med. 2008;102(3):407–12.
Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50(6):1953–8.
Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care. 2006;10(1):R27.
Geller DE. Aerosol antibiotics in cystic fibrosis. Respir Care. 2009;54(5):658–70.
Marchand S, Gobin P, Brillault J, Baptista S, Adier C, Olivier JC, et al. Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats. Antimicrob Agents Chemother. 2010;54(9):3702–7.
Yapa SWS, Li J, Porter CJ, Nation RL, Patel K, McIntosh MP. Population pharmacokinetics of colistin methanesulfonate in rats: achieving sustained lung concentrations of colistin for targeting respiratory infections. Antimicrob Agents Chemother. 2013;57(10):5087–95.
Michalopoulos AS. Aerosolized antibiotics: the past, present and future, with a special emphasis on inhaled colistin. Expert Opin Drug Deliv. 2012;9(5):493–5.
Dudley MN, Loutit J, Griffith DC. Aerosol antibiotics: considerations in pharmacological and clinical evaluation. Curr Opin Biotechnol. 2008;19(6):637–43.
Westerman EM, de Boer AH, Le Brun PP, Touw DJ, Frijlink HW, Heijerman HG. Dry powder inhalation of colistin sulphomethate in healthy volunteers: a pilot study. Int J Pharm. 2007;335(1–2):41–5.
Le Brun PP, de Boer AH, Mannes GP, de Fraiture DM, Brimicombe RW, Touw DJ, et al. Dry powder inhalation of antibiotics in cystic fibrosis therapy: part 2. Inhalation of a novel colistin dry powder formulation: a feasibility study in healthy volunteers and patients. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft Pharm Chem Eng. 2002;54(1):25–32.
Byrne NM, Keavey PM, Perry JD, Gould FK, Spencer DA. Comparison of lung deposition of colomycin using the HaloLite and the Pari LC Plus nebulisers in patients with cystic fibrosis. Arch Dis Child. 2003;88(8):715–8.
Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, et al. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother. 2006;57(2):306–11.
Yapa SW, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJ, Nation RL, McIntosh MP. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob Agents Chemother. 2014.
Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant gram-negative bacteria. Int J Antimicrob Agents. 2005;25(1):11–25.
Li J, Nation RL. Comment on: pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother. 2006;58(1):222–3. author reply 223.
Chua HL, Collis GG, Newbury AM, Chan K, Bower GD, Sly PD, et al. The influence of age on aerosol deposition in children with cystic fibrosis. Eur Respir J. 1994;7(12):2185–91.
Kelly JT, Asgharian B, Wong BA. Inertial particle deposition in a monkey nasal mold compared with that in human nasal replicas. Inhal Toxicol. 2005;17(14):823–30.
Martonen TB, Katz IM, Musante CJ. A nonhuman primate aerosol deposition model for toxicological and pharmaceutical studies. Inhal Toxicol. 2001;13(4):307–24.
Patra AL. Comparative anatomy of mammalian respiratory tracts: the nasopharyngeal region and the tracheobronchial region. J Toxicol Environ Health. 1986;17(2–3):163–74.
Patra AL, Gooya A, Menache MG. A morphometric comparison of the nasopharyngeal airway of laboratory animals and humans. Anat Rec. 1986;215(1):42–50.
Albuquerque-Silva I, Vecellio L, Durand M, Avet J, Le Pennec D, de Monte M, et al. Particle deposition in a child respiratory tract model: in vivo regional deposition of fine and ultrafine aerosols in baboons. PLoS One. 2014;9(4):e95456.
Pitance L, Reychler G, Leal T, Reychler H, Liistro G, Montharu J, et al. Aerosol delivery to the lung is more efficient using an extension with a standard jet nebulizer than an open-vent jet nebulizer. J Aerosol Med Pulm Drug Deliv. 2013;26(4):208–14.
European Pharmacopoeia 8th Edition (8.5). Chapter 2.09.44: preparation for nebulization-characterization. Available from: http://online6edqmeu/ep805/[Website]. 2015.
Dubus JC, Vecellio L, De Monte M, Fink JB, Grimbert D, Montharu J, et al. Aerosol deposition in neonatal ventilation. Pediatr Res. 2005;58(1):10–4.
Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, et al. Consistent global approach on reporting of colistin doses to promote safe and effective use. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;58(1):139–41.
Gagnadoux F, Diot P, Marchand S, Thompson R, Dieckman K, Lemarie E, et al. Pulmonary deposition of colistin aerosols in cystic fibrosis. Comparison of an ultrasonic nebulizer and a pneumatic nebulizer. Rev Mal Respir. 1996;13(1):55–60.
Diot P, Gagnadoux F, Martin C, Ellataoui H, Furet Y, Breteau M, et al. Nebulization and anti-pseudomonas aeruginosa activity of colistin. Eur Respir J. 1997;10(9):1995–8.
Vecellio None L, Grimbert D, Becquemin MH, Boissinot E, Le Pape A, Lemarie E, et al. Validation of laser diffraction method as a substitute for cascade impaction in the European project for a nebulizer standard. J Aerosol Med Off J Int Soc Aerosol Med. 2001;14(1):107–14.
Vecellio None L, Grimbert D, Bordenave J, Benoit G, Furet Y, Fauroux B, et al. Residual gravimetric method to measure nebulizer output. J Aerosol Med Off J Int Soc Aerosol Med. 2004;17(1):63–71.
Pitcairn GR, Newman SP. Tissue attenuation corrections in gamma scintigraphy. J Aerosol Med Off J Int Soc Aerosol Med. 1997;10(3):187–98.
Snell NJ, Ganderton D. Assessing lung deposition of inhaled medications. Consensus statement from a workshop of the British Association for Lung Research, held at the Institute of Biology, London, U.K. on 17 April 1998. Respir Med. 1999;93(2):123–33.
Ilowite JS, Gorvoy JD, Smaldone GC. Quantitative deposition of aerosolized gentamicin in cystic fibrosis. Am Rev Respir Dis. 1987;136(6):1445–9.
Gobin P, Lemaitre F, Marchand S, Couet W, Olivier JC. Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography tandem mass spectrometry (LC-MS/MS). Antimicrob Agents Chemother. 2010;54:1941–8.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34(5):711–26.
Boisson M, Jacobs M, Gregoire N, Gobin P, Marchand S, Couet W, et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother. 2014;58(12):7331–9.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504.
Bulitta JB, Bingolbali A, Shin BS, Landersdorfer CB. Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J. 2011;13(2):201–11.
Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2009.
Karjagin J, Lefeuvre S, Oselin K, Kipper K, Marchand S, Tikkerberi A, et al. Pharmacokinetics of meropenem determined by microdialysis in the peritoneal fluid of patients with severe peritonitis associated with septic shock. Clin Pharmacol Ther. 2008;83(3):452–9.
Dahyot-Fizelier C, Lefeuvre S, Laksiri L, Marchand S, Sawchuk RJ, Couet W, et al. Kinetics of imipenem distribution into the peritoneal fluid of patients with severe peritonitis studied by microdialysis. Clin Pharmacokinet. 2010;49(5):323–34.
Dahyot C, Marchand S, Bodin M, Debeane B, Mimoz O, Couet W. Application of basic pharmacokinetic concepts to analysis of microdialysis data: illustration with imipenem muscle distribution. Clin Pharmacokinet. 2008;47(3):181–9.
Marchand S, Dahyot C, Lamarche I, Mimoz O, Couet W. Microdialysis study of imipenem distribution in skeletal muscle and lung extracellular fluids of noninfected rats. Antimicrob Agents Chemother. 2005;49(6):2356–61.
Marchand S, Forsell A, Chenel M, Comets E, Lamarche I, Couet W. Norfloxacin blood–brain barrier transport in rats is not affected by probenecid coadministration. Antimicrob Agents Chemother. 2006;50(1):371–3.
Lefeuvre S, Marchand S, Pariat C, Lamarche I, Couet W. Microdialysis study of imipenem distribution in the peritoneal fluid of rats with experimental acute pancreatitis. Antimicrob Agents Chemother. 2008;52(4):1516–8.
Dahyot-Fizelier C, Timofeev I, Marchand S, Hutchinson P, Debaene B, Menon D, et al. Brain microdialysis study of meropenem in two patients with acute brain injury. Antimicrob Agents Chemother. 2010;54(8):3502–4.
Dahyot-Fizelier C, Frasca D, Gregoire N, Adier C, Mimoz O, Debaene B, et al. Microdialysis study of cefotaxime cerebral distribution in patients with acute brain injury. Antimicrob Agents Chemother. 2013;57(6):2738–42.
Gontijo AV, Gregoire N, Lamarche I, Gobin P, Couet W, Marchand S. Biopharmaceutical Characterization of Nebulized Antimicrobial Agents in Rats. 2. Colistin. Antimicrob Agents Chemother. 2014.
Gontijo AV, Brillault J, Gregoire N, Lamarche I, Gobin P, Couet W, et al. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 1. Ciprofloxacin, Moxifloxacin, and Grepafloxacin. Antimicrob Agents Chemother. 2014;58(7):3942–9.
Hubert D, Leroy S, Nove-Josserand R, Murris-Espin M, Mely L, Dominique S, et al. Pharmacokinetics and safety of tobramycin administered by the PARI eFlow rapid nebulizer in cystic fibrosis. J Cyst Fibros. 2009;8(5):332–7.
Lu Q, Girardi C, Zhang M, Bouhemad B, Louchahi K, Petitjean O, et al. Nebulized and intravenous colistin in experimental pneumonia caused by pseudomonas aeruginosa. Intensive Care Med. 2010;36(7):1147–55.
Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. Tissue concentrations: do we ever learn? J Antimicrob Chemother. 2008;61(2):235–7.
Kiem S, Schentag JJ. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother. 2008;52(1):24–36.
Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother. 2004;53(5):837–40.
Marchand S, Lamarche I, Gobin P, Couet W. Dose-ranging pharmacokinetics of colistin methanesulphonate (CMS) and colistin in rats following single intravenous CMS doses. J Antimicrob Chemother. 2010;65(8):1753–8.
Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, et al. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther. 2011;89(6):875–9.
Dudhani RV, Turnidge JD, Coulthard K, Milne RW, Rayner CR, Li J, et al. Elucidation of pharmacokinetic/pharmacodynamic determinant of colistin activity against pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother. 2010;54(3):1117–24.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors thank Georges Roseau for its technical assistance in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marchand, S., Bouchene, S., de Monte, M. et al. Pharmacokinetics of Colistin Methansulphonate (CMS) and Colistin after CMS Nebulisation in Baboon Monkeys. Pharm Res 32, 3403–3414 (2015). https://doi.org/10.1007/s11095-015-1716-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1716-0