ABSTRACT
Purpose
The present work focuses on design and development of surface functionalized LCNPs for improving tumor delivery of DTX.
Methods
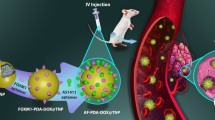
Phytantriol based “stealth” LCNPs were prepared using hydrotrope method and optimized. The potential of developed formulation was further assessed using cell culture experiments, in vivo pharmacokinetics, in vivo pharmacodynamics and toxicity studies.
Results
A biphasic drug release pattern was observed with sustained release of drug till 72 h. In vitro cell culture experiments revealed efficient internalization within MCF-7 cells with 25.80-fold decrease in IC50 value for PEG-LCNPs as compared to free DTX. Mechanistic insights demonstrated preferential co-localization of LCNPs in the vicinity of the nucleus. Furthermore, in vivo pharmacokinetic studies revealed 14.45-fold enhancement in circulation half-life of PEG-LCNPs as compared to marketed formulation Taxotere®. In vivo efficacy studies PEG-LCNPs in DMBA induced breast cancer model revealed ~81% reduction in the tumor burden compared to Taxotere® which caused/achieve only 47% reduction or showed only 47% decrease. Furthermore, safety profile was noted for PEG-LCNPs as compared to Taxotere®, measured as a function of hepato- and nephro-toxicity.
Conclusions
Surface functionalization of LCNPsis a viable approach for improving the therapeutic potential of DTX.







Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under curve
- BUN:
-
Blood urea nitrogen
- CLSM:
-
Confocal laser scanning microscope
- CoQ10:
-
Coenzyme Q 10
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DMBA:
-
7,12-dimethylbenz[a]anthracene
- DSPE:
-
1, 2-Distearoyl-sn-glycero-3-phospho-ethanolamine
- DTX:
-
Docetaxel
- EPR:
-
Enhanced permeation and retention
- FBS:
-
Fetal bovine serum
- FDA:
-
Food and Drugs Administration
- GMO:
-
Glyceryl monooleate
- HBSS:
-
Hanks’s balanced salt solution
- IAEC:
-
Institutional animal ethics committee
- ICH:
-
International conference on harmonization
- LCNPs:
-
Liquid crystalline nanoparticles
- MCF-7:
-
Human adenocarcinoma breast cancer cell line
- MRT:
-
Mean residence time
- MTT:
-
(3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide)
- PDI:
-
Polydispersity index
- PEG:
-
Polyethylene glycol
- SAXS:
-
Small angle X ray spectroscopy
- TEM:
-
Transmission electron microscopy
REFERENCES
Bissery MC, Nohynek G, Sanderink GJ, Lavelle F. Docetaxel (Taxotere): a review of preclinical and clinical experience. Part I: preclinical experience. Anticancer Drugs. 1995;6(3):339–355–63–338.
Cervin C, Tinzl M, Johnsson M, Abrahamsson PA, Tiberg F, Dizeyi N. Properties and effects of a novel liquid crystal nanoparticle formulation of docetaxel in a prostate cancer mouse model. Eur J Pharm Sci. 2010;41(2):369–75.
Baker SD, Li J, ten Tije AJ, Figg WD, Graveland W, Verweij J, et al. Relationship of systemic exposure to unbound docetaxel and neutropenia. Clin Pharmacol Ther. 2005;77(1):43–53.
Engels FK, Sparreboom A, Mathot RA, Verweij J. Potential for improvement of docetaxel-based chemotherapy: a pharmacological review. Br J Cancer. 2005;93(2):173–7.
Guo C, Wang J, Cao F, Lee RJ, Zhai G. Lyotropic liquid crystal systems in drug delivery. Drug Discov Today. 2010;15(23–24):1032–40.
Rizwan SB, Boyd BJ, Rades T, Hook S. Bicontinuous cubic liquid crystals as sustained delivery systems for peptides and proteins. Expert Opin Drug Deliv. 2010;7(10):1133–44.
Swarnakar NK, Thanki K, Jain S. Enhanced antitumor efficacy and counterfeited cardiotoxicity of combinatorial oral therapy using Doxorubicin- and Coenzyme Q10-liquid crystalline nanoparticles in comparison with intravenous Adriamycin. Nanomedicine. 2014;10(6):1231–41.
Spicer PT, Hayden KL, Lynch ML, Ofori-Boateng A, Burns JL. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir. 2001;17(19):5748–56.
Thanki K, Gangwal RP, Sangamwar AT, Jain S. Oral delivery of anticancer drugs: challenges and opportunities. J Control Release. 2013;170(1):15–40.
Drummondand CJ, Fong C. Surfactant self-assembly objects as novel drug delivery vehicles. Curr Opin Colloid Interface Sci. 1999;4(6):449–56.
Lawrence ASC, Bingham A, Capper CB, Hume K. The penetration of water and aqueous soap solutions into fatty substances containing one or two polar groups. J Phys Chem. 1964;68(12):3470–6.
Lancelot A, Sierra T, Serrano JL. Nanostructured liquid-crystalline particles for drug delivery. Expert Opin Drug Deliv. 2014;11(4):547–64.
Barauskas J, Cervin C, Jankunec M, Spandyreva M, Ribokaite K, Tiberg F, et al. Interactions of lipid-based liquid crystalline nanoparticles with model and cell membranes. Int J Pharm. 2010;391(1–2):284–91.
Pan X, Han K, Peng X, Yang Z, Qin L, Zhu C, et al. Nanostructed cubosomes as advanced drug delivery system. Curr Pharm Des. 2013;19(35):6290–7.
Swarnakar NK, Thanki K, Jain S. Bicontinuous cubic liquid crystalline nanoparticles for oral delivery of Doxorubicin: implications on bioavailability, therapeutic efficacy, and cardiotoxicity. Pharm Res. 2014;31(5):1219–38.
Yamashita F, Hashida M. Pharmacokinetic considerations for targeted drug delivery. Adv Drug Deliv Rev. 2013;65(1):139–47.
Kolate A, Baradia D, Patil S, Vhora I, Kore G, Misra A. PEG - a versatile conjugating ligand for drugs and drug delivery systems. J Control Release. 2014;192:67–81.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–84.
Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2014;200(C):138–57.
Jain V, Swarnakar NK, Mishra PR, Verma A, Kaul A, Mishra AK, et al. Paclitaxel loaded PEGylated gleceryl monooleate based nanoparticulate carriers in chemotherapy. Biomaterials. 2012;33(29):7206–20.
Jain AK, Thanki K, Jain S. Solidified self-nanoemulsifying formulation for oral delivery of combinatorial therapeutic regimen: part II in vivo pharmacokinetics, antitumor efficacy and hepatotoxicity. Pharm Res. 2014;31(4):946–58.
Jain S, Jain AK, Pohekar M, Thanki K. Novel self-emulsifying formulation of quercetin for improved in vivo antioxidant potential: Implications for drug-induced cardiotoxicity and nephrotoxicity. Free Radic Biol Med. 2013;65C:117–30.
Swarnakar NK, Thanki K, Jain S. Effect of co-administration of CoQ10-loaded nanoparticles on the efficacy and cardiotoxicity of doxorubicin-loaded nanoparticles. RSC Adv. 2013;3(34):14671–85.
Choi BC, Choi JS, Han HK. Altered pharmacokinetics of paclitaxel by the concomitant use of morin in rats. Int J Pharm. 2006;323(1–2):81–5.
Jain AK, Swarnakar NK, Godugu C, Singh RP, Jain S. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials. 2011;32(2):503–15.
Johnsson M, Lam Y, Barauskas J, Tiberg F. Aqueous phase behavior and dispersed nanoparticles of diglycerol monooleate/glycerol dioleate mixtures. Langmuir. 2005;21(11):5159–65.
Changand C-M, Bodmeier R. Binding of drugs to monoglyceride-based drug delivery systems. Int J Pharm. 1997;147(2):135–42.
Swarnakar NK, Thanki K, Jain S. Lyotropic liquid crystalline nanoparticles of CoQ10: implication of lipase digestibility on oral bioavailability, in vivo antioxidant activity, and in vitro-in vivo relationships. Mol Pharm. 2014;11(5):1435–49.
Vandana M, Sahoo SK. Long circulation and cytotoxicity of PEGylated gemcitabine and its potential for the treatment of pancreatic cancer. Biomaterials. 2010;31(35):9340–56.
Pozzi D, Colapicchioni V, Caracciolo G, Piovesana S, Capriotti AL, Palchetti S, et al. Effect of polyethyleneglycol (PEG) chain length on the bio-nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells. Nanoscale. 2014;6(5):2782–92.
Yang Z, Sahay G, Sriadibhatla S, Kabanov AV. Amphiphilic block copolymers enhance cellular uptake and nuclear entry of polyplex-delivered DNA. Bioconjug Chem. 2008;19(10):1987–94.
Song Q, Wang X, Hu Q, Huang M, Yao L, Qi H, et al. Cellular internalization pathway and transcellular transport of pegylated polyester nanoparticles in Caco-2 cells. Int J Pharm. 2013;445(1–2):58–68.
Panday VRN, Huizing MT, Huinink WWTB, Vermorken JB, Beijnen JH. Hypersensitivity reactions to the taxanes paclitaxel and docetaxel. Clin Drug Investig. 1997;14(5):418–27.
Norris LB, Qureshi ZP, Bookstaver PB, Raisch DW, Sartor O, Chen H, et al. Polysorbate 80 hypersensitivity reactions: a renewed call to action. Commun Oncol. 2010;7(9):425–8.
Takimoto T, Nakabori T, Osa A, Morita S, Terada H, Oseto S, et al. Tubular nephrotoxicity induced by docetaxel in non-small-cell lung cancer patients. Int J Clin Oncol. 2012;17(4):395–8.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors are thankful to Director, NIPER for financial support, necessary infrastructure and facilities. N.K.S and K.T. are grateful to Council of Scientific and Industrial Research (CSIR), GOI, New Delhi, for providing research fellowships. The technical support rendered by Mr. Rahul Mahajan is also duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is a part of Indian Patent Application No. 680/DEL/2013 filed on March 08 2013
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 316 kb)
Rights and permissions
About this article
Cite this article
Jain, S., Bhankur, N., Swarnakar, N.K. et al. Phytantriol Based “Stealth” Lyotropic Liquid Crystalline Nanoparticles for Improved Antitumor Efficacy and Reduced Toxicity of Docetaxel. Pharm Res 32, 3282–3292 (2015). https://doi.org/10.1007/s11095-015-1706-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1706-2