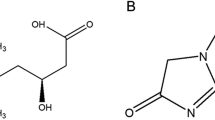
Abstract
Purpose
Since the vitamin D receptor (VDR) was found to up-regulate cerebral P-glycoprotein expression in vitro and in mice, we extend our findings to rats by assessing the effect of rat Vdr activation on brain efflux of quinidine, a P-gp substrate that is eliminated primarily by cytochrome P450 3a.
Methods
We treated rats with vehicle or the active VDR ligand, 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] (4.8 or 6.4 nmol/kg i.p. every 2nd day ×4) and examined P-gp expression and cerebral quinidine disposition via microdialysis in control and treatment studies conducted longitudinally in the same rat.
Results
The 6.4 nmol/kg 1,25(OH)2D3 dose increased cerebral P-gp expression 1.75-fold whereas hepatic Cyp3a remained unchanged. Although there was no change in systemic clearance elicited by 1,25(OH)2D3, brain extracellular fluid quinidine concentrations were lower in treated rats. We noted that insertion of indwelling catheters increased plasma protein binding of quinidine and serial sampling decreased the blood:plasma concentration ratio, factors that alter distribution ratios in microdialysis studies. After appropriate correction, KECF/P,uu and KECF/B,uu, or ratios of quinidine unbound concentrations in brain extracellular fluid to plasma or blood at steady-state, were more than halved.
Conclusion
We demonstrate that VDR activation increases cerebral P-gp expression and delimits brain penetration of P-gp substrates.




Similar content being viewed by others
Abbreviations
- 1,25(OH)2D3 :
-
1α,25-dihydroxyvitamin D3
- aCSF:
-
Artificial cerebrospinal fluid
- BBB:
-
Blood–brain barrier
- Bcrp/BCRP:
-
Rodent/human breast cancer resistance protein
- CAR:
-
Constitutive androstane receptor
- CNS:
-
Central nervous system
- Cyp:
-
Rodent cytochrome P450
- ECF:
-
Extracellular fluid
- Gapdh:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GR:
-
Glucocorticoid receptor
- Mdr1/MDR1:
-
Rodent/human multidrug resistance protein 1
- Mrp/MRP:
-
Rodent/human multidrug resistance-associated protein
- NR:
-
Nuclear receptors
- PBS:
-
Phosphate-buffered saline
- P-gp:
-
P-glycoprotein
- PMSF:
-
Phenylmethylsulfonyl fluoride
- PXR:
-
Pregnane X receptor
- qPCR:
-
Quantitative real-time polymerase chain reaction
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- Vdr/VDR:
-
Rodent/human vitamin D receptor
References
Abbott NJ. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–49.
Lee G, Dallas S, Hong M, Bendayan R. Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol Rev. 2001;53:569–96.
Wang X, Sykes DB, Miller DS. Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood–brain barrier. Mol Pharmacol. 2010;78:376–83.
Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood–brain barrier. Mol Pharmacol. 2004;66:413–9.
Narang VS, Fraga C, Kumar N, Shen J, Throm S, Stewart CF, et al. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood–brain barrier. Am J Physiol Cell Physiol. 2008;295:C440–50.
Chow EC, Durk MR, Cummins CL, Pang KS. 1α,25-Dihydroxyvitamin D3 up-regulates P-glycoprotein via the vitamin D receptor and not farnesoid X receptor in both fxr(−/−) and fxr(+/+) mice and increased renal and brain efflux of digoxin in mice in vivo. J Pharmacol Exp Ther. 2011;337:846–59.
Durk MR, Chan GN, Campos CR, Peart JC, Chow EC, Lee E, et al. 1α,25-Dihydroxyvitamin D3-liganded vitamin D receptor increases expression and transport activity of P-glycoprotein in isolated rat brain capillaries and human and rat brain microvessel endothelial cells. J Neurochem. 2012;123:944–53.
Burris TP, Solt LA, Wang Y, Crumbley C, Banerjee S, Griffett K, et al. Nuclear receptors and their selective pharmacologic modulators. Pharmacol Rev. 2013;65:710–78.
Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16:135–45.
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 α-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30.
Fan J, Liu S, Du Y, Morrison J, Shipman R, Pang KS. Up-regulation of transporters and enzymes by the vitamin D receptor ligands, 1α,25-dihydroxyvitamin D3 and vitamin D analogs, in the Caco-2 cell monolayer. J Pharmacol Exp Ther. 2009;330:389–402.
de Lange EC, Danhof M, de Boer AG, Breimer DD. Critical factors of intracerebral microdialysis as a technique to determine the pharmacokinetics of drugs in rat brain. Brain Res. 1994;666:1–8.
Martignoni M, Groothuis G, de Kanter R. Comparison of mouse and rat cytochrome P450-mediated metabolism in liver and intestine. Drug Metab Dispos. 2006;34:1047–54.
Fremstad D, Jacobsen S, Lunde KM. Influence of serum protein binding on the pharmacokinetics of quinidine in normal and anuric rats. Acta Pharmacol Toxicol (Copenh). 1977;41:161–76.
Teraoand N, Shen DD. Alterations in serum protein binding and pharmacokinetics of l-propranolol in the rat elicited by the presence of an indwelling venous catheter. J Pharmacol Exp Ther. 1983;227:369–75.
Liu L, Mak E, Tirona RG, Tan E, Novikoff PM, Wang P, et al. Vascular binding, blood flow, transporter, and enzyme interactions on the processing of digoxin in rat liver. J Pharmacol Exp Ther. 2005;315:433–48.
de Lange EC, Danhof M, de Boer AG, Breimer DD. Methodological considerations of intracerebral microdialysis in pharmacokinetic studies on drug transport across the blood–brain barrier. Brain Res Brain Res Rev. 1997;25:27–49.
Chan GN, Saldivia V, Yang Y, Pang H, de Lannoy I, Bendayan R. In vivo induction of P-glycoprotein expression at the mouse blood–brain barrier: an intracerebral microdialysis study. J Neurochem. 2013;127:342–52.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Jonesand G, Tenenhouse HS. 1,25(OH)2D, the preferred substrate for CYP24. J Bone Miner Res. 2002;17:179–81.
Chow EC, Maeng HJ, Liu S, Khan AA, Groothuis GM, Pang KS. 1α,25-Dihydroxyvitamin D3 triggered vitamin D receptor and farnesoid X receptor-like effects in rat intestine and liver in vivo. Biopharm Drug Dispos. 2009;30:457–75.
Sugihara N, Furuno K, Kita N, Murakami T, Yata N. Distribution of quinidine in rats with carbon tetrachloride-intoxicated hepatic disease. J Pharmacobiodyn. 1992;15:167–74.
Pollack GM, Brouwer KL, Demby KB, Jones JA. Determination of hepatic blood flow in the rat using sequential infusions of indocyanine green or galactose. Drug Metab Dispos. 1990;18:197–202.
Sato Y, Asoh T, Oizumi K. High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer’s disease. Bone. 1998;23:555–7.
Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14:1032–40.
Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65:1348–52.
Hollo A, Clemens Z, Kamondi A, Lakatos P, Szucs A. Correction of vitamin D deficiency improves seizure control in epilepsy: a pilot study. Epilepsy Behav. 2012;24:131–3.
Kalueff AV, Minasyan A, Keisala T, Kuuslahti M, Miettinen S, Tuohimaa P. Increased severity of chemically induced seizures in mice with partially deleted Vitamin D receptor gene. Neurosci Lett. 2006;394:69–73.
Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-Dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343:139–43.
Orme RP, Bhangal MS, Fricker RA. Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE. 2013;8:e62040.
Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21:98–108.
Zanatta L, Goulart PB, Goncalves R, Pierozan P, Winkelmann-Duarte EC, Woehl VM, et al. 1alpha,25-dihydroxyvitamin D(3) mechanism of action: modulation of L-type calcium channels leading to calcium uptake and intermediate filament phosphorylation in cerebral cortex of young rats. Biochim Biophys Acta. 2012;1823:1708–19.
Kim RB. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev. 2002;34:47–54.
Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, et al. β-Amyloid efflux mediated by p-glycoprotein. J Neurochem. 2001;76:1121–28.
Hammarlund-Udenaes M. Active-site concentrations of chemicals - are they a better predictor of effect than plasma/organ/tissue concentrations? Basic Clin Pharmacol Toxicol. 2010;106:215–20.
Shuaib A, Xu K, Crain B, Siren AL, Feuerstein G, Hallenbeck J, et al. Assessment of damage from implantation of microdialysis probes in the rat hippocampus with silver degeneration staining. Neurosci Lett. 1990;112:149–54.
de Lange EC, Danhof M, Zurcher C, de Boer AG, Breimer DD. Repeated microdialysis perfusions: periprobe tissue reactions and BBB permeability. Brain Res. 1995;702:261–5.
de Lange EC, de Bock G, Schinkel AH, de Boer AG, Breimer DD. BBB transport and P-glycoprotein functionality using MDR1A (−/−) and wild-type mice. Total brain versus microdialysis concentration profiles of rhodamine-123. Pharm Res. 1998;15:1657–65.
Liu X, Van Natta K, Yeo H, Vilenski O, Weller PE, Worboys PD, et al. Unbound drug concentration in brain homogenate and cerebral spinal fluid at steady state as a surrogate for unbound concentration in brain interstitial fluid. Drug Metab Dispos. 2009;37:787–93.
Syvanen S, Schenke M, van den Berg DJ, Voskuyl RA, de Lange EC. Alteration in P-glycoprotein functionality affects intrabrain distribution of quinidine more than brain entry-a study in rats subjected to status epilepticus by kainate. AAPS J. 2012;14:87–96.
Doran A, Obach RS, Smith BJ, Hosea NA, Becker S, Callegari E, et al. The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: evaluation using the MDR1A/1B knockout mouse model. Drug Metab Dispos. 2005;33:165–74.
Schmiedlin-Ren P, Thummel KE, Fisher JM, Paine MF, Lown KS, Watkins PB. Expression of enzymatically active CYP3A4 by Caco-2 cells grown on extracellular matrix-coated permeable supports in the presence of 1α,25-dihydroxy vitamin D3. Mol Pharmacol. 1997;51:741–54.
Khan AA, Chow EC, van Loenen-Weemaes AM, Porte RJ, Pang KS, Groothuis GM. Comparison of effects of VDR versus PXR, FXR and GR ligands on the regulation of CYP3A isozymes in rat and human intestine and liver. Eur J Pharm Sci. 2009;37:115–25.
Cong D, Doherty M, Pang KS. A new physiologically based, segregated-flow model to explain route-dependent intestinal metabolism. Drug Metab Dispos. 2000;28:224–35.
Woodland C, Huang TT, Gryz E, Bendayan R, Fawcett JP. Expression, activity and regulation of CYP3A in human and rodent brain. Drug Metab Rev. 2008;40:149–68.
Chow EC, Sun H, Khan AA, Groothuis GM, Pang KS. Effects of 1α,25-dihydroxyvitamin D3 on transporters and enzymes of the rat intestine and kidney in vivo. Biopharm Drug Dispos. 2010;31:91–108.
M. Rodriguez, J.R. Munoz-Castaneda, Y. Almaden. Therapeutic Use Of Calcitriol. Curr Vasc Pharmacol (2013).
Muindi JR, Peng Y, Potter DM, Hershberger PA, Tauch JS, Capozzoli MJ, et al. Pharmacokinetics of high-dose oral calcitriol: results from a phase 1 trial of calcitriol and paclitaxel. Clin Pharmacol Ther. 2002;72:648–59.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors have no conflict of interest to declare. This work was supported by the Canadian Institutes of Health Research (CIHR) and by NoAb BioDiscoveries (NoAb) and InterVivo Solutions (IVS). Matthew R. Durk was supported by a CIHR Strategic Training Grant in Biological Therapeutics and a Pfizer Canada Graduate Scholarship in Science and Technology. Additionally, we wish to thank employees of IVS, Sophie Pan and Julia Izhakova, for the bioanalysis of the microsomal, protein binding and blood; plasma ratio study samples and Victor Saldivia for carrying out the blood: plasma ratio studies. David K. H. Lee (NoAb and IVS) is thanked for approval of our collaborative efforts.
Author information
Authors and Affiliations
Corresponding author
Additional information
MRD and JF contributed equally as first authors and K. Sandy Pang and Inés A.M. de Lannoy are equal co-senior authors
Rights and permissions
About this article
Cite this article
Durk, M.R., Fan, J., Sun, H. et al. Vitamin D Receptor Activation Induces P-Glycoprotein and Increases Brain Efflux of Quinidine:An Intracerebral Microdialysis Study in Conscious Rats. Pharm Res 32, 1128–1140 (2015). https://doi.org/10.1007/s11095-014-1524-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1524-y