Abstract
Purpose
We investigated the RESS process as a means of simultaneous micronization and cocrystallization of a model drug with poor aqueous solubility.
Methods
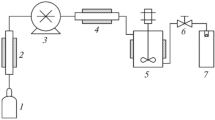
1:1 cocrystals of ibuprofen (IBU) and nicotinamide (NA) were produced with a pilot scale unit for RESS processing.IBU and NA were dissolved in scCO2 at 30 MPa and 50°C. After 24 h, the supercritical solution was expanded at a medium CO2 flow rate of 3.8 kg/h during 60 min into an expansion vessel kept at ambient conditions. Cocrystals were identified with DSC, XRD and confocal Raman microscopy (CRM) and further characterized by SEM, specific surface area, wetting ability, solubility and dissolution testing.
Results
Judging by DSC, XRD and CRM, cocrystals with high purity could be produced with the RESS technique. Micronization via RESS was successful, since the specific surface area of RESS cocrystals was increased almost tenfold in comparison to cocrystals produced by slow solvent evaporation. Due to the additional micronization, the mean dissolution time of IBU from RESS cocrystals was decreased.
Conclusions
RESS cocrystallization offers the advantage of combining micronization and cocrystallization in a single production step. For drugs with dissolution-limited bioavailability, RESS cocrystallization may therefore be a superior approach in comparison to established cocrystallization techniques.








Similar content being viewed by others
Abbreviations
- IBU:
-
Ibuprofen
- NA:
-
Nicotinamide
- RCC 0.5:1:
-
RESS coprecipitates with a molar ratio of 0.5:1 (IBU:NA)
- RCC 1:1:
-
RESS coprecipitates with a molar ratio of 1:1 (IBU:NA)
- SCC 1:1:
-
Cocrystals produced by slow solvent evaporation
- ScCO2 :
-
Supercritical carbon dioxide
References
Elder DP, Holm R, Diego HL. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int J Pharm. 2013;453(1):88–100.
U.S. Food and Drug Administration 2013. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Regulatory Classification of Pharmaceutical Cocrystals.
Ainouz A, Authelin J-R, Billot P, Lieberman H. Modeling and prediction of cocrystal phase diagrams. Int J Pharm. 2009;374(1–2):82–9.
Chow SF, Chen M, Shi L, Chow AHL, Sun CC. Simultaneously improving the mechanical properties, dissolution performance, and hygroscopicity of ibuprofen and flurbiprofen by cocrystallization with nicotinamide. Pharm Res. 2012;29(7):1854–65.
Debenedetti PG, Tom JW, Kwauk X, Yeo SD. Rapid expansion of supercritical solutions (RESS): fundamentals and applications. Fluid Phase Equilib. 1993;82:311–21.
Hirunsit P, Huang Z, Srinophakun T, Charoenchaitrakool M, Kawi S. Particle formation of ibuprofen-supercritical CO2 system from rapid expansion of supercritical solutions (RESS): a mathematical model. Powder Technol. 2005;154(2–3):83–94.
Türk M. Formation of small organic particles by RESS: experimental and theoretical investigations. J Supercrit Fluids. 1999;15(1):79–89.
Türk M, Hils P, Helfgen B, Schaber K, Martin HJ, Wahl MA. Micronization of pharmaceutical substances by the Rapid Expansion of Supercritical Solutions (RESS): a promising method to improve bioavailability of poorly soluble pharmaceutical agents. J Supercrit Fluids. 2002;22(1):75–84.
Ginty PJ, Whitaker MJ, Shakesheff KM, Howdle SM. Drug delivery goes supercritical. Mater Today. 2005;8(8):42–8.
Vemavarapu C, Mollan MJ, Lodaya M, Needham TE. Design and process aspects of laboratory scale SCF particle formation systems. Int J Pharm. 2005;292(1):1–16.
Constable DJC, Jimenez-Gonzalez C, Henderson RK. Perspective on solvent use in the pharmaceutical industry. Org Process Res Dev. 2006;11(1):133–7.
Pasquali I, Bettini R, Giordano F. Solid-state chemistry and particle engineering with supercritical fluids in pharmaceutics. Eur J Pharm Sci. 2006;27(4):299–310.
Pasquali I, Bettini R. Are pharmaceutics really going supercritical? Int J Pharm. 2008;364(2):176–87.
Jung J, Perrut M. Particle design using supercritical fluids: literature and patent survey. J Supercrit Fluids. 2001;20(3):179–219.
Wahl MA. Supercritical fluid technology. In: Parikh DM, editor. Handbook of pharmaceutical granulation technology. New York: Informa Healthcare; 2009. p. 126–37.
Hezave AZ, Esmaeilzadeh F. Micronization of drug particles via RESS process. J Supercrit Fluids. 2010;52(1):84–98.
Kayrak D, Akman U, Hortaçsu Ö. Micronization of ibuprofen by RESS. J Supercrit Fluids. 2003;26(1):17–31.
Vemavarapu C, Mollan MJ, Needham TE. Coprecipitation of pharmaceutical actives and their structurally related additives by the RESS process. Powder Technol. 2009;189(3):444–53.
Johannsen M, Brunner G. Measurements of solubilities of xanthines in supercritical carbon dioxide + methanol. J Chem Eng Data. 1995;40(2):431–4.
Padrela L, Rodrigues MA, Velaga SP, Matos HA, de Azevedo EG. Formation of indomethacin-saccharin cocrystals using supercritical fluid technology. Eur J Pharm Sci. 2009;38(1):9–17.
Charoenchaitrakool M, Dehghani F, Foster NR, Chan HK. Micronization by rapid expansion of supercritical solutions to enhance the dissolution rates of poorly water-soluble pharmaceuticals. Ind Eng Chem Res. 2000;39(12):4794–802.
Oberoi LM, Alexander KS, Riga AT. Study of interaction between ibuprofen and nicotinamide using differential scanning calorimetry, spectroscopy, and microscopy and formulation of a fast-acting and possibly better ibuprofen suspension for osteoarthritis patients. J Pharm Sci. 2004;94(1):93–101.
Berry DJ, Seaton CC, Clegg W, Harrington RW, Coles SJ, Horton PN, et al. Applying hot-stage microscopy to co-crystal screening: a study of nicotinamide with seven active pharmaceutical ingredients. Cryst Growth Des. 2008;8(5):1697–712.
Dhumal RS, Kelly AL, York P, Coates PD, Paradkar A. Cocrystalization and simultaneous agglomeration using hot melt extrusion. Pharm Res. 2010;27(12):2725–33.
Kelly AL, Gough T, Dhumal RS, Halsey SA, Paradkar A. Monitoring ibuprofen-nicotinamide cocrystal formation during solvent free continuous cocrystallization (SFCC) using near infrared spectroscopy as a PAT tool. Int J Pharm. 2012;426(1–2):15–20.
Kotnik P, Škerget M, Knez Ž. Solubility of nicotinic acid and nicotinamide in carbon dioxide at T = (313.15 to 373.15) K and p = (5 to 30) MPa: experimental data and correlation. J Chem Eng Data. 2011;56(2):338–43.
Sheridan PL, Buckton G, Storey DE. The extent of errors associated with contact angles II. factors affecting data obtained using a Wilhelmy plate technique for powders. Int J Pharm. 1994;109(2):155–71.
Grossjohann C, Eccles KS, Maguire AR, Lawrence SE, Tajber L, Corrigan OI, et al. Characterisation, solubility and intrinsic dissolution behaviour of benzamide: dibenzyl sulfoxide cocrystal. Int J Pharm. 2012;422(1–2):24–32.
Akalin E, Akyuz S. Vibrational analysis of free and hydrogen bonded complexes of nicotinamide and picolinamide. Vib Spectrosc. 2006;42(2):333–40.
Rossi B, Verrocchio P, Viliani G, Mancini I, Guella G, Rigo E, et al. Vibrational properties of ibuprofen–cyclodextrin inclusion complexes investigated by Raman scattering and numerical simulation. J Raman Spectrosc. 2009;40(4):453–8.
Jubert A, Legarto ML, Massa NE, Tévez LL, Okulik NB. Vibrational and theoretical studies of non-steroidal anti-inflammatory drugs Ibuprofen [2-(4-isobutylphenyl)propionic acid]; Naproxen [6-methoxy-α ± −methyl-2-naphthalene acetic acid] and Tolmetin acids [1-methyl-5-(4-methylbenzoyl)-1H-pyrrole-2-acetic acid]. J Mol Struct. 2006;783(1–3):34–51.
Truelove J, Bawarshi-Nassar R, Chen NR, Hussain A. Solubility enhancement of some developmental anti-cancer nucleoside analogs by complexation with nicotinamide. Int J Pharm. 1984;19(1):17–25.
Sanghvi R, Evans D, Yalkowsky SH. Stacking complexation by nicotinamide: a useful way of enhancing drug solubility. Int J Pharm. 2007;336(1):35–41.
Matson DW, Fulton JL, Petersen RC, Smith RD. Rapid expansion of supercritical fluid solutions: solute formation of powders, thin films, and fibers. Ind Eng Chem Res. 1987;26(11):2298–306.
ACKNOWLEDGMENTS AND DISCLOSURES
A part of this work has been presented as a poster at the AAPS Annual Meeting in San Antonio, US, Nov. 10–14, 2013 and at the 9th PBP world meeting in Lisbon, Portugal, March 31—April 3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müllers, K.C., Paisana, M. & Wahl, M.A. Simultaneous Formation and Micronization of Pharmaceutical Cocrystals by Rapid Expansion of Supercritical Solutions (RESS). Pharm Res 32, 702–713 (2015). https://doi.org/10.1007/s11095-014-1498-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1498-9