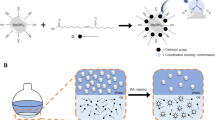
Abstract
Purpose
: To develop a bio-assay for measuring long-term bioactivity of released anti-inflammatory compounds and to test the bioactivity of celecoxib (CXB) and triamcinolone acetonide (TA) released from a new PLGA-based microsphere platform.
Methods
: Human osteoarthritic chondrocytes were plated according to standardized procedures after batch-wise harvest and cultured for 3 days to prevent cell confluency and changes in cell behaviour. Prostaglandin E2 (PGE2) production stimulated by TNFα was used as a parameter of inflammation. A novel microsphere platform based on PTE-functionalised PLGA was used to incorporate CXB and TA. Loaded microspheres were added to transwells overlying the cells, with transfer of the wells to new cell cultures every 3 days. Inhibition of PGE2 production was determined over a period of 21 days.
Results
: PLGA(75:25)-PTE microspheres were prepared and loaded with CXB and TA at 86 and 97% loading efficiency, respectively. In the bioactivity assay, PGE2 levels induced by TNFα were reduced to an average of 30% using microspheres loaded with 0.1 nmol CXB per transwell; with microspheres loaded with 0.1 nmol TA, PGE2 production was initially reduced to 3% and gradually recovered to 30% reduction. At 1 nmol loading, PGE2 was inhibited to 0–7% for CXB-loaded microspheres, and 0–28% for TA-loaded microspheres.
Conclusions
: We present a novel sustained release bioactivity assay which provides an essential link between in vitro buffer-based release kinetics and in vivo application. Novel PLGA-based microspheres loaded with TA and CXB showed efficient anti-inflammatory effects over time.






Similar content being viewed by others
Abbreviations
- CXB:
-
Celecoxib
- Mn:
-
Number-average molecular weight
- Mw:
-
Weight-average molecular weight
- OA:
-
Osteoarthritis
- PBS:
-
Phosphate buffered saline
- PDI:
-
Polydispersity index
- PGE2 :
-
Prostaglandin E2
- PLGA-PTE:
-
Poly(lactic-co-glycolic acid) with poly(thioester) linkages
- TA:
-
Triamcinolone acetonide
- TNFα:
-
Tumor necrosis factor alpha
Reference
The burden of musculoskeletal diseases at the start of the new millennium. World Health Organization. 2003; Technical Report Series no 919.
Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35.
Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120–30.
Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976). 2006;31(23):2724–7.
Clark AG, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the johnston county osteoarthritis project. Arthritis Rheum. 1999;42(11):2356–64.
Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol. 2007;53(5):4–18.
Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American college of rheumatology subcommittee on osteoarthritis guidelines. Arthritis Rheum. 2000;43(9):1905–15.
Roberts S, Butler RC. Inflammatory mediators as potential therapeutic targets in the spine. Curr Drug Targets Inflamm Allergy. 2005;4(2):257–66.
Solomon SD, Pfeffer MA, McMurray JJ, Fowler R, Finn P, Levin B, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114(10):1028–35.
Saag KG, Koehnke R, Caldwell JR, Brasington R, Burmeister LF, Zimmerman B, et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994;96(2):115–23.
Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract. 2006;12(4):358–62.
Gaffney K, Ledingham J, Perry JD. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis. 1995;54(5):379–81.
Heyneman CA, Lawless-Liday C, Wall GC. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000;60(3):555–74.
Pyne D, Ioannou Y, Mootoo R, Bhanji A. Intra-articular steroids in knee osteoarthritis: a comparative study of triamcinolone hexacetonide and methylprednisolone acetate. Clin Rheumatol. 2004;23(2):116–20.
Cao P, Jiang L, Zhuang C, Yang Y, Zhang Z, Chen W, et al. Intradiscal injection therapy for degenerative chronic discogenic low back pain with end plate modic changes. Spine J. 2011;11(2):100–6.
Lopez-Garcia F, Vazquez-Auton JM, Gil F, Latoore R, Moreno F, Villalain J, et al. Intra-articular therapy of experimental arthritis with a derivative of triamcinolone acetonide incorporated in liposomes. J Pharm Pharmacol. 1993;45(6):576–8.
Thakkar H, Sharma RK, Mishra AK, Chuttani K, Murthy RS. Celecoxib incorporated chitosan microspheres: in vitro and in vivo evaluation. J Drug Target. 2004;12(9–10):549–57.
de Silva M, Hazleman BL, Thomas DP, Wraight P. Liposomes in arthritis: a new approach. Lancet. 1979;1(8130):1320–2.
Rowland M. Plasma protein binding and therapeutic drug monitoring. Ther Drug Monit. 1980;2(1):29–37.
Hoyle CE, Lee TY, Roper T. Thiol–enes: chemistry of the past with promise for the future. J Polym Sci Part A: Pol Chem. 2004;42(21):5301–38.
van Dijk M, Rijkers DTS, Liskamp RMJ, van Nostrum CF, Hennink WE. Synthesis and applications of biomedical and pharmaceutical polymers via click chemistry methodologies. Bioconjug Chem. 2009;20(11):2001–16.
Dias A, Boerakker M, Nijenhuis A. Polymers comprising thioester bonds. 2007:WO/2007/028612.
Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. J Control Release. 2003;90(3):261–80.
Baboota S, Faiyaz S, Ahuja A, Ali J, Shafiq S, Ahmad S. Development and validation of a stability-indicating HPLC method for analysis of Celecoxib (CXB) in bulk drug and microemulsion formulations. Acta Chromatogr. 2007;18:116–29.
Ahn JS, Choi HK, Chun MK, Ryu JM, Jung JH, Kim YU, et al. Release of triamcinolone acetonide from mucoadhesive polymer composed of chitosan and poly(acrylic acid) in vitro. Biomaterials. 2002;23(6):1411–6.
Goldie I, Nachemson A. Synovial pH in rheumatoid knee-joints. I. eff synovectomy Acta Orthop Scand. 1969;40(5):634–41.
Nachemson A. Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop Scand. 1969;40(1):23–42.
Chandran S, Ravi P, Saha RN. Development and in vitro evaluation of oral controlled release formulations of celecoxib using optimization techniques. Yakugaku zasshi: J e Pharm Soc Jpn. 2006;126(7):505–14.
Center for Drug Evaluation and Research. Approval package for: application number ANDA 090164 “ Triamcinolone Acetonide Injectable Suspension USP 40 mg/mL”. FDA. 2009.
van Diest PJ. No consent should be needed for using leftover body material for scientific purposes. For Bmj. 2002;325(7365):648–51.
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–22.
DrugBank database. Celecoxib (DB00482). http://www.drugbank.ca/.
Royal Society of Chemistry. Tiamcinolone acetonide. http://www.rsc.org/learn-chemistry.
Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems–a review. Int J Pharm. 2011;415(1–2):34–52.
Faisant N, Akiki J, Siepmann F, Benoit JP, Siepmann J. Effects of the type of release medium on drug release from PLGA-based microparticles: experiment and theory. Int J Pharm. 2006;314(2):189–97.
Seedher N, Bhatia S. Mechanism of interaction of the non-steroidal antiinflammatory drugs meloxicam and nimesulide with serum albumin. J Pharm Biomed Anal. 2005;39(1–2):257–62.
Kempen DH, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29(22):3245–52.
Lu L, Garcia CA, Mikos AG. In vitro degradation of thin poly(DL-lactic-co-glycolic acid) films. J Biomed Mater Res. 1999;46(2):236–44.
Witschi C, Doelker E. Influence of the microencapsulation method and peptide loading on poly(lactic acid) and poly(lactic-co-glycolic acid) degradation during in vitro testing. J Control Release. 1998;51(2–3):327–41.
Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21(23):2475–90.
Cui F, Cun D, Tao A, Yang M, Shi K, Zhao M, et al. Preparation and characterization of melittin-loaded poly (DL-lactic acid) or poly (DL-lactic-co-glycolic acid) microspheres made by the double emulsion method. J Control Release. 2005;107(2):310–9.
Hazekawa M, Sakai Y, Yoshida M, Haraguchi T, Uchida T. Single injection of ONO-1301-loaded PLGA microspheres directly after ischaemia reduces ischaemic damage in rats subjected to middle cerebral artery occlusion. J Pharm Pharmacol. 2012;64(3):353–9.
Cheng S, Afif H, Martel-Pelletier J, Pelletier JP, Li X, Farrajota K, et al. Activation of peroxisome proliferator-activated receptor gamma inhibits interleukin-1beta-induced membrane-associated prostaglandin E2 synthase-1 expression in human synovial fibroblasts by interfering with Egr-1. J Biol Chem. 2004;279(21):22057–65.
Kirtikara K, Morham SG, Raghow R, Laulederkind SJ, Kanekura T, Goorha S, et al. Compensatory prostaglandin E2 biosynthesis in cyclooxygenase 1 or 2 null cells. J Exp Med. 1998;187(4):517–23.
Moore RA, Derry S, Makinson GT, McQuay HJ. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports. Arthritis Res Ther. 2005;7(3):R644–665.
Hollander J, Brown EMJ, Jessar RA, Brown CY. Hydrocortisone and cortisone injected into arthritic joints: Comparative effects of and use of hydrocortisone as a local antiarthritic agent. J Am Med Assoc. 1951;147(17):1629.
Hollander JL, Jessar RA, Brown EMJ. Intra-synovial corticosteroid therapy: a decade of use. Bull Rheum Dis. 1961;11:239–40.
Dieppe PA, Sathapatayavongs B, Jones HE, Bacon PA, Ring EF. Intra-articular steroids in osteoarthritis. Rheum Rehabil. 1980;19(4):212–7.
Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370–7.
Jean YH, Wen ZH, Chang YC, Hsieh SP, Tang CC, Wang YH, et al. Intra-articular injection of the cyclooxygenase-2 inhibitor parecoxib attenuates osteoarthritis progression in anterior cruciate ligament-transected knee in rats: role of excitatory amino acids. Osteoarthr Cartil. 2007;15(6):638–45.
Lee JW, Choi SW, Park SH, Lee GY, Kang HS. MR-based outcome predictors of lumbar transforaminal epidural steroid injection for lumbar radiculopathy caused by herniated intervertebral disc. Eur Radiol. 2013;23(1):205–11.
Papavasiliou AV, Isaac DL, Marimuthu R, Skyrme A, Armitage A. Infection in knee replacements after previous injection of intra-articular steroid. J Bone Joint Surg (Br). 2006;88(3):321–3.
Kaspar S. de VdBJ. Infection in hip arthroplasty after previous injection of steroid. J Bone Joint Surg (Br). 2005;87(4):454–7.
Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364(2):298–327.
Laeschke K. Biocompatibility of microparticles into soft tissue fillers. Semin cutaneous med surg. 2004;23(4):214–7.
Acknowledgments AND DISCLOSURES
This research forms part of the Project P2.01 IDiDAS of the research program of the BioMedical Materials institute, co-funded by the Dutch Ministry of Economic Affairs. The financial contribution of the Dutch Arthritis Association is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Hy., van Dijk, M., Licht, R. et al. Applicability of a Newly Developed Bioassay for Determining Bioactivity of Anti-Inflammatory Compounds in Release Studies − Celecoxib and Triamcinolone Acetonide Released from Novel PLGA-Based Microspheres . Pharm Res 32, 680–690 (2015). https://doi.org/10.1007/s11095-014-1495-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1495-z