Abstract
Purpose
The aim of this study was to investigate influencing factors on the dissolution test for powders for pulmonary delivery with USP apparatus 2 (paddle apparatus).
Methods
We investigated the influence of dose collection method, membrane holder type and the presence of surfactants on the dissolution process. Furthermore, we modeled the in vitro dissolution process to identify influencing factors on the dissolution process of inhaled formulations based on the Nernst-Brunner equation.
Results
A homogenous distribution of the powder was required to eliminate mass dependent dissolution profiles. This was also found by modeling the dissolution process under ideal conditions. Additionally, it could be shown that influence on the diffusion pathway depends on the solubility of the substance.
Conclusion
We demonstrated that the use of 0.02% DPPC in the dissolution media results in the most discriminating and reproducible dissolution profiles.
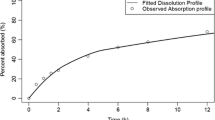
In the model section we demonstrated that the dissolution process depends strongly on saturation solubility and particle size. Under defined assumptions we were able show that the model is predicting the experimental dissolution profiles.









Similar content being viewed by others
Abbreviations
- aACI:
-
Abbreviated Andersen cascade impactor
- ACI:
-
Andersen cascade impactor
- ACN:
-
Acetonitrile
- API:
-
Active pharmaceutical ingredient
- DPPC:
-
Dipalmytoylphosphatidylcholine
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- FPD:
-
Fine particle dose
- HPLC:
-
High performance liquid chromatography
- mACI:
-
Abbreviated Andersen cascade impactor with stage extension and modified filter stage
- PBS:
-
Phosphate buffered saline
- RC:
-
Regenerated cellulose membrane
- RP:
-
Reversed phase
- SDS:
-
Sodium dodecyl sulfate
- SE:
-
Stage extension
- SEM:
-
Scanning electron microscopy
- USP:
-
United States Pharmacopoeia
- ρ:
-
Density
- ηwater :
-
Dynamic viscosity of water at 37°C
- cs :
-
Solubility of drug
- ct :
-
Concentration of the drug in the solution at time t
- D:
-
Diffusion coefficient of substance in the solvent
- daero :
-
Aerodynamic particle diameter
- dgeo :
-
Geometric particle diameter
- dm:
-
Mass of solid material at time t
- dt:
-
Time interval
- f1 :
-
Difference factor
- f2 :
-
Similarity factor
- h:
-
Diffusion (boundary) layer thickness
- k:
-
Shape factor
- m:
-
Amount of drug released
- Ne :
-
Number of particles in a particle size fraction
- r:
-
Radius
- Rt :
-
Mean percent drug released at each time point for reference product
- S:
-
The surface area of the particles
- Se :
-
The surface area of each particle size fraction
- t:
-
Time
- Tt :
-
Mean percent drug released at each time point for test product
- V:
-
Volume
- VM :
-
Van der Waals volume
- Xe (0):
-
The amount of undissolved drug in a particle size group
- Xe(t):
-
The amount of undissolved drug in a particle size group e
- Xsum(t):
-
Total amount of undissolved drug at time t
References
Gray VA, Hickey AJ, Balmer P, Davies NM, Dunbar C, Foster TS, et al. The inhalation ad hoc advisory panel for the USP performance tests of inhalation dosage forms. Pharmacopeial Forum. 2008;34:1068–74.
United States Pharmacopeial Convention. Dissolution. In United States Pharamacopeia and National Formulary, Rockville, 2011.
United States Pharmacopeial Convention. Drug release. In United States Pharmacopeia and National Formulary, Rockville, 2013.
United States Pharmacopeial Convention. Aerosol, nasal sprays, metered dose inhalers, and dry powder inhaler. In United States Pharmacopeia and National Formulary, Maryland, 2012.
Labouta HI, Schneider M. Tailor-made biofunctionalized nanoparticles using layer-by-layer technology. Int J Pharm. 2010;395:236–42.
Riley T, Christopher D, Arp J, Casazza A, Colombani A, Cooper A, et al. Challenges with developing in vitro dissolution tests for orally inhaled products (OIPs). AAPS PharmSciTech. 2012;13:978–89.
May S, Jensen B, Wolkenhauer M, Schneider M, Lehr CM. Dissolution techniques for in vitro testing of dry powders for inhalation. Pharm Res. 2012;29:2157–66.
Salama RO, Traini D, Chan HK, Young PM. Preparation and characterisation of controlled release co-spray dried drug-polymer microparticles for inhalation 2: evaluation of in vitro release profiling methodologies for controlled release respiratory aerosols. Eur J Pharm Biopharm. 2008;70:145–52.
Haghi M, Traini D, Bebawy M, Young PM. Deposition, diffusion and transport mechanism of dry powder microparticulate salbutamol, at the respiratory epithelia. Mol Pharm. 2012;9:1717–26.
Arora D, Shah KA, Halquist MS, Sakagami M. In vitro aqueous fluid-capacity-limited dissolution testing of respirable aerosol drug particles generated from inhaler products. Pharm Res. 2010;27:786–95.
Son YJ, Horng M, Copley M, McConville JT. Optimization of an in vitro dissolution test method for inhalation formulations. Dissolution Technol. 2010;17:6–13.
Davies NM, Feddah MR. A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm. 2003;255:175–87.
Sakagami M, Arora Lakhani D. Understanding dissolution in the presence of competing cellular uptake and absorption in the airways. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, Young PM, editors. Respiratory Drug Delivery. 2012, pp. 185–192.
Mees J, Fulton C, Wilson S, Bramwell N, Lucius M, Cooper A. Development of dissolution methodology for dry powder inhalation aerosols. IPAC-RS Conference In 2011.
Veldhuizen R, Nag K, Orgeig S, Possmayer F. The role of lipids in pulmonary surfactant. Biochim Biophys Acta Mol basis Dis. 1998;1408:90–108.
Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the Biopharmaceutics classification system. Int J Pharm. 2006;321:1–11.
Hsu W-L, Lin M-J, Hsu J-P. Dissolution of solid particle in liquids: a shrinking core model. World Acad Sci Eng Technol. 2009;53:913–8.
Wang J, Flanagan DR. General solution for diffusion-controlled dissolution of spherical particles. 1. Theory. J Pharm Sci. 1999;88:731–8.
Son YJ, McConville JT. Development of a standardized dissolution test method for inhaled pharmaceutical formulations. Int J Pharm. 2009;382:15–22.
Jensen B, Reiners M, Wolkenhauer M, Ritzheim P, May S, Schneider M, et al. Dissolution testing for inhaled products. Respiratory Drug Delivery, Europe In 2011, pp. 303–308.
May S, Jensen B, Wolkenhauer M, Schneider M, Lehr CM. Impact of deposition and the presence of surfactants on in vitro dissolution of inhalation powders. Respiratory Drug Delivery Europe In 2013.
Food and Drug Administration. Guidance for Industry; Dissolution testing of immediate release solid oral dosage forms. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070237.pdf online (1997).
European Medicines Agency. Guideline on the investigation of bioequivalence. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf online (2008).
Sertsou G. Analytical derivation of time required for dissolution of monodisperse drug particles. J Pharm Sci. 2004;93:1941–4.
Hayduk W, Laudie H. Prediction of diffusion coefficients for nonelectrolytes in dilute aqueous solutions. AIChe. 1974;20:611–5.
Sheng JJ, Sirois PJ, Dressman JB, Amidon GL. Particle diffusional layer thickness in a USP dissolution apparatus II: a combined function of particle size and paddle speed. J Pharm Sci. 2008;97:4815–29.
Bisrat M, Nystrom C. Physicochemical aspects of drug release. VIII. The relation between particle size and surface specific dissolution rate in agitated suspensions. Int J Pharm. 1988;47:223–31.
Lu ATK, Frisella ME, Johnson KC. Dissolution modeling: factors affecting the dissolution rates of polydisperse powders. Pharm Res. 1993;10:1308–14.
Hintz RJ, Johnson KC. The effect of particle size distribution on dissolution rate and oral absorption. Int J Pharm. 1989;51:9–17.
Sugano K. Theoretical comparison of hydrodynamic diffusion layer models used for dissolution simulation in drug discovery and development. Int J Pharm. 2008;363:73–7.
Voigt R. Pharmazeutische Technologie. Stuttgart: Deutscher Apotheker Verlag; 2006.
Nichols SC. Calibration and mensuration issues for the standard and modified andersen cascade impactor. Pharmacopeial Forum. 2000;26:1466–7.
Okazaki A, Mano T, Sugano K. Theoretical dissolution model of poly-disperse drug particles in biorelevant media. J Pharm Sci. 2008;97:1843–52.
Davies CN. Particle-fluid interaction. J Aerosol Sci. 1979;10:477–513.
http://www.guidechem.com/dictionary/de/51333-22-3.html. 2013.
Sadler RC, Prime D, Burnell PK, Martin GP, Forbes B. Integrated in vitro experimental modelling of inhaled drug delivery: deposition, dissolution and absorption. J Drug Deliv Sci Technol. 2011;21:331–8.
Wauthoz N, Deleuze P, Saumet A, Duret C, Kiss R, Amighi K. Temozolomide-based dry powder formulations for lung tumor-related inhalation treatment. Pharm Res. 2011;28:762–75.
ACKNOWLEDGMENTS AND DISCLOSURES
Thanks to Dr. Holger Wagner, Dr. Peter Häbel and team (Boehringer Ingelheim) for calculating the van der Waals volumes of the substances and to Wolfgang Bootz (Boehringer Ingelheim) and Dr. Bernhard Meier for the SEM pictures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
May, S., Jensen, B., Weiler, C. et al. Dissolution Testing of Powders for Inhalation: Influence of Particle Deposition and Modeling of Dissolution Profiles. Pharm Res 31, 3211–3224 (2014). https://doi.org/10.1007/s11095-014-1413-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1413-4