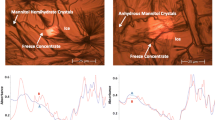
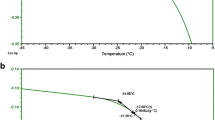
Abstract
Purpose
We hypothesize that ultrasonication can accelerate solute crystallization in freeze-concentrates. Our objective is to demonstrate ultrasonication as a potential predictive tool for evaluating physical stability of excipients in frozen solutions.
Methods
The crystallization tendencies of lyoprotectants (trehalose, sucrose), carboxylic acid buffers (citric, tartaric, malic, and acetic) and an amino acid buffer (histidine HCl) were studied. Aqueous solutions of buffers, lyoprotectants and mixtures of the two were cooled from room temperature to −20°C and sonicated to induce solute crystallization. The crystallized phases were identified by X-ray diffractometry (laboratory or synchrotron source).
Results
Sonication accelerated crystallization of trehalose dihydrate in frozen trehalose solutions. Sonication also enhanced solute crystallization in tartaric (200 mM; pH 5), citric (200 mM pH 4) and malic (200 mM; pH 4) acid buffers. At lower buffer concentrations, longer annealing times following sonication were required to facilitate solute crystallization. The time for crystallization of histidine HCl progressively increased as a function of sucrose concentration. The insonation period required to effect crystallization also increased with sucrose concentration.
Conclusions
Sonication can substantially accelerate solute crystallization in the freeze-concentrate. Ultrasonication may be useful in assessing the crystallization tendency of formulation constituents used in long term frozen storage and freeze-drying.








Similar content being viewed by others
References
Singh SK, Kolhe P, Wang W, Nema S. Large-scale freezing of biologics. Bio Proc Int. 2009;7:32–44.
Jameel F, Padala C, Randolph TW. Strategies for bulk storage and shipment of proteins. Formulation and process development strategies for manufacturing biopharmaceuticals. John Wiley & Sons, Inc., 2010, pp. 677-704.
Piedmonte D, Summers C, McAuley A, Karamujic L, Ratnaswamy G. Sorbitol crystallization can lead to protein aggregation in frozen protein formulations. Pharm Res. 2007;24:136–46.
Singh S, Kolhe P, Mehta A, Chico S, Lary A, Huang M. Frozen state storage instability of a monoclonal antibody: aggregation as a consequence of trehalose crystallization and protein unfolding. Pharm Res. 2011;28:873–85.
Sundaramurthi P, Shalaev E, Suryanarayanan R. Calorimetric and diffractometric evidence for the sequential crystallization of buffer components and consequent pH swing in frozen solutions. J Phys Chem Lett. 2010;114:4915–23.
Pikal-Cleland KA, Rodríguez-Hornedo N, Amidon GL, Carpenter JF. Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric [beta]-Galactosidase. Arch Biochem Biophys. 2000;384:398–406.
Lam XM, Costantino HR, Overcashier DE, Nguyen TH, Hsu CC. Replacing succinate with glycolate buffer improves the stability of lyophilized interferon-γ. Int J Pharm. 1996;142:85–95.
Ni P, Ding J, Rao B. In situ cryogenic Raman spectroscopic studies on the synthetic fluid inclusions in the systems H2O and NaCl-H2O. Chin Sci Bull. 2006;51:108–14.
Cho H, Shepson PB, Barrie LA, Cowin JP, Zaveri R. NMR Investigation of the quasi-brine layer in ice/brine mixtures. J Phys Chem B. 2002;106:11226–32.
Murase N, Franks F. Salt precipitation during the freeze-concentration of phosphate buffer solutions. Biophys Chem. 1989;34:293–300.
Kim AI, Akers MJ, Nail SL. The physical state of mannitol after freeze-drying: effects of mannitol concentration, freezing rate, and a noncrystallizing cosolute. J Pharm Sci. 1998;87:931–5.
Sundaramurthiand P, Suryanarayanan R. Influence of crystallizing and non-crystallizing cosolutes on trehalose crystallization during freeze-drying. Pharm Res. 2010;27:2384–93.
Carless JE. Accelerated tests for physical stability of pharmaceutical products. Pestic Sci. 1970;1:270–3.
Fischer P, Eugster A, Windhab EJ, Schuleit M. Predictive stress tests to study the influence of processing procedures on long term stability of supersaturated pharmaceutical o/w creams. Int J Pharm. 2007;339:189–96.
Lucas TI, Bishara RH, Seevers RH. A stability program for the distribution of drug products. Pharm Technol. 2004;28:68–73.
Ferreira C, Crespo R, Martins PM, Gales L, Rocha F, Damas AM. Small temperature oscillations promote protein crystallization. Cryst Eng Comm. 2011;13:3051–6.
Kordylla A, Koch S, Tumakaka F, Schembecker G. Towards an optimized crystallization with ultrasound: effect of solvent properties and ultrasonic process parameters. J Cryst Growth. 2008;310:4177–84.
Kordylla A, Krawczyk T, Tumakaka F, Schembecker G. Modeling ultrasound-induced nucleation during cooling crystallization. Chem Eng Sci. 2009;64:1635–42.
Ichitsubo T, Matsubara E, Yamamoto T, Chen HS, Nishiyama N, Saida J, et al. Microstructure of fragile metallic glasses inferred from ultrasound-accelerated crystallization in Pd-based metallic glasses. Phys Rev Lett. 2005;95:245501.
Zheng L, Sun D-W. Innovative applications of power ultrasound during food freezing processes—a review. Trends Food SciTechnol. 2006;17:16–23.
Ruecroft G, Hipkiss D, Ly T, Maxted N, Cains PW. Sonocrystallization: the use of ultrasound for improved industrial crystallization. Org Proc Res Dev. 2005;9:923–32.
Kim S, Wei C, Kiang S. Crystallization process development of an active pharmaceutical ingredient and particle engineering via the use of ultrasonics and temperature cycling. Org Proc Res Dev. 2003;7:997–1001.
Nakagawa K, Hottot A, Vessot S, Andrieu J. Influence of controlled nucleation by ultrasounds on ice morphology of frozen formulations for pharmaceutical proteins freeze-drying. Chem Eng Proc : Process Intensific. 2006;45:783–91.
Powder Diffraction File. histidine HCl monohydrate, Card # 00-054-1737; DL-malic acid, Card # 00-031-1776; Na hydrogen malate, Card # 00-051-2464, Na citrate monohydrate, Card # 00-043-1524; Na acetate trihydrate, Card # 00-028-1030., International Centre for Diffraction Data, Newtown Square, PA, 2004.
Varshney D, Kumar S, Shalaev E, Sundaramurthi P, Kang S-W, Gatlin L, et al. Glycine crystallization in frozen and freeze-dried systems: effect of pH and buffer concentration. Pharm Res. 2007;24:593–604.
Beyer KD, Schroeder JR, Pearson CS. Solid/Liquid phase diagram of the ammonium Sulfate/Maleic Acid/Water System. J Phys Chem A. 2011;115:13842–51.
Banker GS, Siepmann J, Rhodes C. Modern pharmaceutics, CRC Press, 2002.
Green W. The “melting-point” of hydrated sodium acetate: solubility curves. J Phys Chem. 1908;12:655–60.
Dahms A. Nachträge und Bemerkungen zu der Arbeit über Gefrierpunkte binärer Gemenge. Annalen der Physik. 1896;296:119–23.
Izutsu K-i, Yoshioka S, Terao T. Decreased protein-stabilizing effects of cryoprotectants due to crystallization. Pharm Res. 1993;10:1232–7.
Crowe LM, Reid DS, Crowe JH. Is trehalose special for preserving dry biomaterials? Biophys J. 1996;71:2087–93.
Coutinho C, Bernardes E, Félix D, Panek AD. Trehalose as cryoprotectant for preservation of yeast strains. J Biotechnol. 1988;7:23–32.
Akers MJ. Excipient–drug interactions in parenteral formulations. J Pharm Sci. 2002;91:2283–300.
Sundaramurthiand P, Suryanarayanan R. Trehalose crystallization during freeze-drying: implications on lyoprotection. J Phys Chem Lett. 2010;1:510–4.
Miller DP, de Pablo JJ, Corti H. Thermophysical properties of trehalose and its concentrated aqueous solutions. Pharm Res. 1997;14:578–90.
Sundaramurthi P, Patapoff TW, Suryanarayanan R. Crystallization of trehalose in the frozen solutions and its phase behavior during drying. Pharm Res. 2010;27:2374–83.
Luque de Castro MD, Priego-Capote F. Ultrasound-assisted crystallization (sonocrystallization). Ultrason Sonochem. 2007;14:717–24.
Young FE, Jones FT. Sucrose hydrates. The sucrose–water phase diagram. J Physic Coll Chem. 1948;53:1334–50.
Roos YH. Chapter 5 - food components and polymers. San Diego: Phase Transitions in Foods, Academic Press; 1995. p. 109–56.
Sundaramurthiand P, Suryanarayanan R. Thermophysical properties of carboxylic and amino acid buffers at subzero temperatures: relevance to frozen state stabilization. J Phys Chem B. 2011;115:7154–64.
Kolhe P, Amend E, Singh SK. Impact of freezing on pH of buffered solutions and consequences for monoclonal antibody aggregation. Biotechnol Prog. 2010;26:727–33.
Nail SL, and Gatlin LA. Freeze-drying: Principles and practice. Pharmaceutical Dosage Forms: Parenteral Medications, pp. 353-382.
Gómez G, Pikal MJ, Rodríguez-Hornedo N. Effect of initial buffer composition on pH changes during far-from-equilibrium freezing of sodium phosphate buffer solutions. Pharm Res. 2001;18:90–7.
Cho H, Shepson PB, Barrie LA, Cowin JP, Zaveri R. NMR investigation of the Quasi-Brine layer in Ice/Brine Mixtures. J Phys Chem B. 2002;106:11226–32.
Muraseand N, Franks F. Salt precipitation during the freeze-concentration of phosphate buffer solutions. Biophys Chem. 1989;34:293–300.
Sundaramurthiand P, Suryanarayanan R. The effect of crystallizing and non-crystallizing cosolutes on succinate buffer crystallization and the consequent pH shift in frozen solutions. Pharm Res. 2011;28:374–85.
van den Bergand L, Rose D. Effect of freezing on the pH and composition of sodium and potassium phosphate solutions: the reciprocal system KH2PO4–Na2HPO4–H2O. Arch Biochem Biophys. 1959;81:319–29.
Varshney D, Kumar S, Shalaev E, Kang S-W, Gatlin L, Suryanarayanan R. Solute crystallization in frozen systems–use of synchrotron radiation to improve sensitivity. Pharm Res. 2006;23:2368–74.
Acknowledgments and Disclosures
The work was partially supported by the William and Mildred Peters endowment fund. Parts of this work were carried out in the Characterization Facility, University of Minnesota, which receives partial support from NSF through the MRSEC program. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ragoonanan, V., Suryanarayanan, R. Ultrasonication as a Potential Tool to Predict Solute Crystallization in Freeze-Concentrates. Pharm Res 31, 1512–1524 (2014). https://doi.org/10.1007/s11095-013-1257-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1257-3