Abstract
Purpose
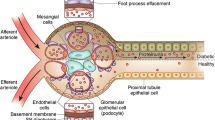
This study employed a mouse model to evaluate the effects of diabetic nephropathy on the pharmacokinetics of 8C2, a murine monoclonal antibody (mAb).
Methods
Streptozotocin (STZ) was administered to mice to induce diabetic nephropathy (125 mg/kg/day × 2). Mice were grouped (n = 8–10) based on time after STZ-treatment (control, 1, 2, 3, 4, or 6 weeks), and injected intravenously with 10 mg/kg 8C2. Blood samples were collected up to 7 days, and 8C2 plasma concentrations were determined via immunoassay. Inulin clearance and urinary albumin excretion rate (UAE) were determined to assess renal function.
Results
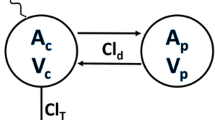
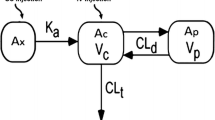
UAE, inulin clearance, and 8C2 clearance increased significantly following STZ. Comparing control and 6 week STZ-treatment groups, UAE and inulin clearance increased from 25.7 ± 3.3 to 99.3 ± 13.7 μg/day, and from 421 ± 31 to 584 ± 78 μl/min. 8C2 clearance increased from 121 ± 12.5 to 228 ± 61 μl/hr/kg (p < 0.01). 8C2 clearance was highly correlated with UAE (r2: 0.731). Inclusion of UAE as a covariate in population modeling explained significant residual variability in 8C2 clearance.
Conclusions
The clearance of 8C2 increased significantly in STZ-treated mice. Population pharmacokinetic modeling suggests that UAE has potential for use in predicting mAb clearance in subjects with diabetic nephropathy, possibly assisting in the individualization of mAb dosing.





Similar content being viewed by others
Abbreviations
- CL:
-
systemic clearance
- GFR:
-
glomerular filtration rate
- IgG:
-
immunoglobulin G
- mAb:
-
monoclonal antibody
- STZ:
-
streptozotocin
- UAE:
-
urinary albumin excretion rate
REFERENCES
Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21–39.
Reichert JM. Metrics for antibody therapeutics development. MAbs. 2010;2:695–700.
Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3:944–55.
McCarthy KM, Lam M, Subramanian L, Shakya R, Wu Z, Newton EE. Effects of mutations in potential phosphorylation sites on transcytosis of FcRn. J Cell Sci. 2001;114:1591–8.
Antohe F, Radulescu L, Gafencu A, Ghetie V, Simionescu M. Expression of functionally active FcRn and the differentiated bidirectional transport of IgG in human placental endothelial cells. Hum Immunol. 2001;62:93–105.
Praetor A, Ellinger I, Hunziker W. Intracellular traffic of the MHC class I-like IgG Fc receptor, FcRn, expressed in epithelial MDCK cells. J Cell Sci. 1999;112(Pt 14):2291–9.
Ward ES, Zhou J, Ghetie V, Ober RJ. Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int Immunol. 2003;15:187–95.
Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93:5512–6.
Raghavan M, Chen MY, Gastinel LN, Bjorkman PJ. Investigation of the interaction between the class I MHC-related Fc receptor and its immunoglobulin G ligand. Immunity. 1994;1:303–15.
Brambell FW, Hemmings WA, Morris IG. A theoretical model of gamma-globulin catabolism. Nature. 1964;203:1352–4.
Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–8.
Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56:248–52.
Vugmeyster Y, Xu X, Theil FP, Khawli LA, Leach MW. Pharmacokinetics and toxicology of therapeutic proteins: advances and challenges. World J Biol Chem. 2012;3:73–92.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–59.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–5.
Crommelin DJA, Sindelar RD. Pharmaceutical biotechnology: an introduction for pharmacists and pharmaceutical scientists. London; New York: Routledge; 2002.
Woo KT, Lau YK. Proteinuria: clinical significance and basis for therapy. Singap Med J. 2001;42:385–9.
Bakoush O, Tencer J, Tapia J, Rippe B, Torffvit O. Higher urinary IgM excretion in type 2 diabetic nephropathy compared to type 1 diabetic nephropathy. Kidney Int. 2002;61:203–8.
Lemley KV, Blouch K, Abdullah I, Boothroyd DB, Bennett PH, Myers BD, et al. Glomerular permselectivity at the onset of nephropathy in type 2 diabetes mellitus. J Am Soc Nephrol. 2000;11:2095–105.
Mistry K, Kalia K. Non enzymatic glycosylation of IgG and their urinary excretion in patients with diabetic nephropathy. Indian J Clin Biochem. 2009;24:159–65.
Zhu Y, Hu C, Lu M, Liao S, Marini JC, Yohrling J, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL-12/23p40, in patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2009;49:162–75.
Chen J, Lu Q, Balthasar JP. Mathematical modeling of topotecan pharmacokinetics and toxicodynamics in mice. J Pharmacokinet Pharmacodyn. 2007;34:829–47.
Shah D. Pharmacokinetic strategies to improve the safety and efficacy of intraperitoneal chemotherapy, Pharmaceutical Sciences, Vol. Doctor of Philosophy, University at Buffalo 2010, p. 585.
Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, et al. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286:F590–6.
Lixsoft. Modelling & Simulation for Drug Development. http://www.monolix.org/ (accessed 2013 March 12 2013).
Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, et al. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes. 2005;54:2628–37.
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–76.
Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. New Engl J Med. 1999;341:1127–33.
Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26:2392–9.
Tarnow L, Rossing P, Nielsen FS, Fagerudd JA, Poirier O, Parving HH. Cardiovascular morbidity and early mortality cluster in parents of type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2000;23:30–3.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
Herman WH, Zimmet P. Type 2 diabetes: an epidemic requiring global attention and urgent action. Diabetes Care. 2012;35:943–4.
Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32 Suppl 2:64–78.
Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28.
Bakris GL. Recognition, pathogenesis, and treatment of different stages of nephropathy in patients with type 2 diabetes mellitus. Mayo Clin Proc. 2011;86:444–56.
Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol. 2006;290:F214–22.
Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes. 2000;49:1399–408.
Chow FY, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in streptozotocin-induced diabetic nephropathy: potential role in renal fibrosis. Nephrol Dial Transplant. 2004;19:2987–96.
Guyton AC, Hall JE. Textbook of medical physiology. Philadelphia: Elsevier Saunders; 2006.
Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–52.
McQuarrie EP, Shakerdi L, Jardine AG, Fox JG, Mackinnon B. Fractional excretions of albumin and IgG are the best predictors of progression in primary glomerulonephritis. Nephrol Dial Transplant. 2011;26:1563–9.
Narita T, Fujita H, Koshimura J, Meguro H, Kitazato H, Shimotomai T, et al. Glycemic control reverses increases in urinary excretions of immunoglobulin G and ceruloplasmin in type 2 diabetic patients with normoalbuminuria. Horm Metab Res. 2001;33:370–8.
Deckert T, Feldt-Rasmussen B, Djurup R, Deckert M. Glomerular size and charge selectivity in insulin-dependent diabetes mellitus. Kidney Int. 1988;33:100–6.
Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–53.
Meneton P, Ichikawa I, Inagami T, Schnermann J. Renal physiology of the mouse. Am J Physiol Renal Physiol. 2000;278:F339–51.
Meyer MH, Meyer Jr RA, Gray RW, Irwin RL. Picric acid methods greatly overestimate serum creatinine in mice: more accurate results with high-performance liquid chromatography. Anal Biochem. 1985;144:285–90.
Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, et al. Nephropathy in diabetes. Diabetes Care. 2004;27:S79–83.
Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117–25.
ACKNOWLEDGMENTS AND DISCLOSURES
This study was supported by a grant from the Center for Protein Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engler, F.A., Zheng, B. & Balthasar, J.P. Investigation of the Influence of Nephropathy on Monoclonal Antibody Disposition: A Pharmacokinetic Study in a Mouse Model of Diabetic Nephropathy. Pharm Res 31, 1185–1193 (2014). https://doi.org/10.1007/s11095-013-1241-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1241-y