ABSTRACT
Purpose
To investigate the effects of normothermic hepatic ischemia-reperfusion (IR) injury on the activity of P-glycoprotein (P-gp) in the liver and at the blood–brain barrier (BBB) of rats using rhodamine 123 (RH-123) as an in vivo marker.
Methods
Rats were subjected to 90 min of partial ischemia or sham surgery, followed by 12 or 24 h of reperfusion. Following intravenous injection, the concentrations of RH-123 in blood, bile, brain, and liver were used for pharmacokinetic calculations. The protein levels of P-gp and some other transporters in the liver and brain were also determined by Western blot analysis.
Results
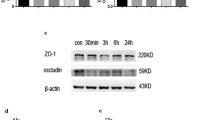
P-gp protein levels at the liver canalicular membrane were increased by twofold after 24 h of reperfusion. However, the biliary excretion of RH-123 was reduced in these rats by 26%, presumably due to IR-induced reductions in the liver uptake of the marker and hepatic ATP concentrations. At the BBB, a 24% overexpression of P-gp in the 24-h IR animals was associated with a 30% decrease in the apparent brain uptake clearance of RH-123. The pharmacokinetics or brain distribution of RH-123 was not affected by the 12-h IR injury.
Conclusions
Hepatic IR injury may alter the peripheral pharmacokinetics and brain distribution of drugs that are transported by P-gp and possibly other transporters.






Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- AUC:
-
Area under the plasma concentration-time curve
- BBB:
-
Blood–brain barrier
- BIBF:
-
Bile salt-independent bile flow
- C 40 br :
-
Concentration in the brain at 40 min
- Cliver :
-
Concentration in the liver
- Cmedian :
-
Concentration in the median lobe of the liver
- Cright :
-
Concentration in the right lobe of the liver
- DTT:
-
DL-dithiothreitol
- f u :
-
Unbound fraction in plasma
- IPRL:
-
Isolated perfused rat liver
- IR:
-
Ischemia-reperfusion
- K in :
-
Apparent brain uptake clearance
- LPS:
-
Lipopolysaccharides
- Mrp:
-
Multidrug resistance-associated protein
- Oatp:
-
Organic anion transporting polypeptide
- P-gp:
-
P-glycoprotein
- PMSF:
-
Phenylmethanesulfonyl fluoride
- RH-110:
-
Rhodamine 110
- RH-123:
-
Rhodamine 123
- RH-Glu:
-
Rhodamine glucuronide
- TNF- α:
-
Tumor necrosis factor-α
- UDPGA:
-
Uridine 5′-diphosphoglucuronic acid
- Wischemic :
-
Weight of ischemic lobes of the liver
- Wliver :
-
Total weight of the liver
- Wnon-ischemic :
-
Weight of non-ischemic lobes of the liver
REFERENCES
Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327–38.
Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26.
Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–9.
Fiorini RN, Shafizadeh SF, Polito C, Rodwell DW, Cheng G, Evans Z, et al. Anti-endotoxin monoclonal antibodies are protective against hepatic ischemia/reperfusion injury in steatotic mice. Am J Transplant. 2004;4:1567–73.
Wanner GA, Ertel W, Muller P, Hofer Y, Leiderer R, Menger MD, et al. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock. 1996;5:34–40.
Rymsa B, Wang JF, de Groot H. O2-. release by activated Kupffer cells upon hypoxia-reoxygenation. Am J Physiol. 1991;261:G602–7.
Liu P, McGuire GM, Fisher MA, Farhood A, Smith CW, Jaeschke H. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock. 1995;3:56–62.
Tanaka Y, Chen C, Maher JM, Klaassen CD. Kupffer cell-mediated downregulation of hepatic transporter expression in rat hepatic ischemia-reperfusion. Transplantation. 2006;82:258–66.
Fardel O, Le Vee M. Regulation of human hepatic drug transporter expression by pro-inflammatory cytokines. Expert Opin Drug Metab Toxicol. 2009;5:1469–81.
Geier A, Dietrich CG, Voigt S, Kim SK, Gerloff T, Kullak-Ublick GA, et al. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology. 2003;38:345–54.
Hartmann G, Cheung AK, Piquette-Miller M. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J Pharmacol Exp Ther. 2002;303:273–81.
Ikemura K, Urano K, Matsuda H, Mizutani H, Iwamoto T, Okuda M. Decreased oral absorption of cyclosporine A after liver ischemia-reperfusion injury in rats: the contribution of CYP3A and P-glycoprotein to the first-pass metabolism in intestinal epithelial cells. J Pharmacol Exp Ther. 2009;328:249–55.
Tanaka Y, Chen C, Maher JM, Klaassen CD. Ischemia-reperfusion of rat livers decreases liver and increases kidney multidrug resistance associated protein 2 (Mrp2). Toxicol Sci. 2008;101:171–8.
Fouassier L, Beaussier M, Schiffer E, Rey C, Barbu V, Mergey M, et al. Hypoxia-induced changes in the expression of rat hepatobiliary transporter genes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G25–35.
Parasrampuria R, Shaik IH, Mehvar R. Effects of in vivo hepatic ischemia-reperfusion injury on the hepatobiliary disposition of rhodamine 123 and its metabolites in isolated perfused rat livers. J Pharm Pharm Sci. 2012;15:318–28.
Syvanen S, Lindhe O, Palner M, Kornum BR, Rahman O, Langstrom B, et al. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab Dispos. 2009;37:635–43.
Agarwal S, Elmquist WF. Insight into the cooperation of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) at the blood-brain barrier: a case study examining sorafenib efflux clearance. Mol Pharm. 2012;9:678–84.
Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31:246–54.
Kusuhara H, Sugiyama Y. Efflux transport systems for drugs at the blood-brain barrier and blood-cerebrospinal fluid barrier (Part 2). Drug Discov Today. 2001;6:206–12.
Syvanen S, Xie R, Sahin S, Hammarlund-Udenaes M. Pharmacokinetic consequences of active drug efflux at the blood-brain barrier. Pharm Res. 2006;23:705–17.
Ando H, Nishio Y, Ito K, Nakao A, Wang L, Zhao YL, et al. Effect of endotoxin on P-glycoprotein-mediated biliary and renal excretion of rhodamine-123 in rats. Antimicrob Agents Chemother. 2001;45:3462–7.
Wang Q, Yang H, Miller DW, Elmquist WF. Effect of the p-glycoprotein inhibitor, cyclosporin A, on the distribution of rhodamine-123 to the brain: an in vivo microdialysis study in freely moving rats. Biochem Biophys Res Commun. 1995;211:719–26.
de Lange EC, de Bock G, Schinkel AH, de Boer AG, Breimer DD. BBB transport and P-glycoprotein functionality using MDR1A (-/-) and wild-type mice. Total brain versus microdialysis concentration profiles of rhodamine-123. Pharm Res. 1998;15:1657–65.
Stapf V, Thalhammer T, Huber-Huber R, Felberbauer F, Gajdzik L, Graf J. Inhibition of rhodamine 123 secretion by cyclosporin A as a model of P-glycoprotein mediated transport in liver. Anticancer Res. 1994;14:581–5.
Parasrampuria R, Mehvar R. Effects of P-glycoprotein and Mrp2 inhibitors on the hepatobiliary disposition of Rhodamine 123 and its glucuronidated metabolite in isolated perfused rat livers. J Pharm Sci. 2010;99:455–66.
Shaik IH, Mehvar R. Cytochrome P450 induction by phenobarbital exacerbates warm hepatic ischemia-reperfusion injury in rat livers. Free Radic Res. 2010;44:441–53.
Spiegel HU, Bahde R. Experimental models of temporary normothermic liver ischemia. J Investig Surg. 2006;19:113–23.
Shaik IH, Mehvar R. Effects of normothermic hepatic ischemia-reperfusion injury on the in vivo, isolated perfused liver, and microsomal disposition of chlorzoxazone, a cytochrome P450 2E1 probe, in rats. J Pharm Sci. 2011;100:5281–92.
Sweatman TW, Seshadri R, Israel M. Metabolism and elimination of rhodamine 123 in the rat. Cancer Chemother Pharmacol. 1990;27:205–10.
Parasrampuria R, Mehvar R. Hepatobiliary disposition of rhodamine 123 in isolated perfused rat livers. Xenobiotica. 2008;38:1263–73.
Lee KJ, Mower R, Hollenbeck T, Castelo J, Johnson N, Gordon P, et al. Modulation of nonspecific binding in ultrafiltration protein binding studies. Pharm Res. 2003;20:1015–21.
Manfredi G, Yang L, Gajewski CD, Mattiazzi M. Measurements of ATP in mammalian cells. Methods. 2002;26:317–26.
Vuppugalla R, Mehvar R. Selective effects of nitric oxide on the disposition of chlorzoxazone and dextromethorphan in isolated perfused rat livers. Drug Metab Dispos. 2006;34:1160–6.
Killard AJ, O’Kennedy R, Bogan DP. Analysis of the glucuronidation of 7-hydroxycoumarin by HPLC. J Pharm Biomed Anal. 1996;14:1585–90.
Bickel U. How to measure drug transport across the blood-brain barrier. NeuroRx. 2005;2:15–26.
Bedirli N, Ofluoglu E, Kerem M, Utebey G, Alper M, Yilmazer D, et al. Hepatic energy metabolism and the differential protective effects of sevoflurane and isoflurane anesthesia in a rat hepatic ischemia-reperfusion injury model. Anesth Analg. 2008;106:830–7.
Ofluoglu E, Kerem M, Pasaoglu H, Turkozkan N, Seven I, Bedirli A, et al. Delayed energy protection of ischemic preconditioning on hepatic ischemia/reperfusion injury in rats. Eur Surg Res. 2006;38:114–21.
Annaert PP, Brouwer KL. Assessment of drug interactions in hepatobiliary transport using rhodamine 123 in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2005;33:388–94.
Forster S, Thumser AE, Hood SR, Plant N. Characterization of rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PLoS ONE. 2012;7:e33253.
Ban D, Kudo A, Sui S, Tanaka S, Nakamura N, Ito K, et al. Decreased Mrp2-dependent bile flow in the post-warm ischemic rat liver. J Surg Res. 2009;153:310–6.
Omae T, Goto M, Shimomura M, Masuda S, Ito K, Okuda M, et al. Transient up-regulation of P-glycoprotein reduces tacrolimus absorption after ischemia-reperfusion injury in rat ileum. Biochem Pharmacol. 2005;69:561–8.
Hartz AM, Bauer B, Fricker G, Miller DS. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–70.
Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–75.
Rigor RR, Hawkins BT, Miller DS. Activation of PKC isoform beta(I) at the blood-brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J Cereb Blood Flow Metab. 2010;30:1373–83.
Peralta C, Fernandez L, Panes J, Prats N, Sans M, Pique JM, et al. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology. 2001;33:100–13.
Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102:1677–90.
McCaffrey G, Staatz WD, Sanchez-Covarrubias L, Finch JD, Demarco K, Laracuente ML, et al. P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem. 2012; 122:962–75.
Ose A, Kusuhara H, Endo C, Tohyama K, Miyajima M, Kitamura S, et al. Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab Dispos. 2010;38:168–76.
Ronaldson PT, Finch JD, Demarco KM, Quigley CE, Davis TP. Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J Pharmacol Exp Ther. 2011;336:827–39.
Liu X, Yang Z, Yang J, Yang H. Increased P-glycoprotein expression and decreased phenobarbital distribution in the brain of pentylenetetrazole-kindled rats. Neuropharmacology. 2007;53:657–63.
ACKNOWLEDGMENTS AND DISCLOSURES
Mohammad K. Miah and Imam H. Shaik contributed equally to this work. The authors would like to acknowledge financial support from the Blood–brain Barrier Research Center at Texas Tech School of Pharmacy. Additionally, we would like to thank Dr. Thomas J. Thekkumkara for the use of VersaDoc Image System in his laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miah, M.K., Shaik, I.H., Bickel, U. et al. Effects of Hepatic Ischemia-Reperfusion Injury on the P-Glycoprotein Activity at the Liver Canalicular Membrane and Blood–Brain Barrier Determined by In Vivo Administration of Rhodamine 123 in Rats. Pharm Res 31, 861–873 (2014). https://doi.org/10.1007/s11095-013-1208-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1208-z