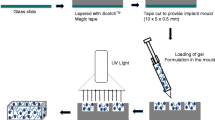
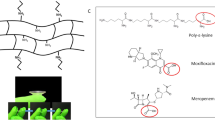
ABSTRACT
Purpose
An autofeedback complex polymeric platform was used in the design of an intelligent intraocular implant—the I3—using stimuli-responsive polymers, producing a smart release system capable of delivering therapeutic levels of an anti-inflammatory agent (indomethacin) and antibiotic (ciprofloxacin) for posterior segment disorders of the eye in response to inflammation.
Methods
Physicochemical and physicomechanical analysis of the I3 was undertaken to explicate the highly crosslinked make-up and ‘on-off’ inflammation-responsive performance of the I3. In addition, energetic profiles for important complexation reactions were generated using Molecular Mechanics Energy Relationships by exploring the spatial disposition of energy minimized molecular structures. Furthermore, preliminary in vivo determination of the inflammation-responsiveness of the I3 was ascertained following implantation in the normal and inflamed rabbit eye.
Results
In silico modeling simulating a pathological inflammatory intraocular state highlighted the interaction potential of hydroxyl radicals with the selected polysaccharides comprising the I3. The intricately crosslinked polymeric system forming the I3 thus responded at an innate level predicted by its molecular make-up to inflammatory conditions as indicated by the results of the drug release studies, rheological analysis, magnetic resonance imaging and scanning electron microscopic imaging. In vivo drug release analysis demonstrated indomethacin levels of 0.749 ± 0.126 μg/mL and 1.168 ± 0.186 μg/mL, and ciprofloxacin levels of 1.181 ± 0.150 μg/mL and 6.653 ± 0.605 μg/mL in the normal and inflamed eye, respectively.
Conclusions
Extensive in vitro, molecular, and in vivo characterization therefore highlighted successful inflammation-responsiveness of the I3. The I3 is a proposed step forward from other described ocular systems owing to its combined bioresponsive, nano-enabled architecture.










Similar content being viewed by others
Abbreviations
- ALG:
-
Alginate
- BPMs:
-
Bioresponsive polymeric matrices
- CMV:
-
Cytomegalovirus
- DCC:
-
N,N′-dicyclohexylcarbodiimide
- DSPC:
-
Distearoylphosphatidylcholine
- DSPE:
-
Distearoylphosphatidylethanolamine
- HA:
-
Hyaluronic acid
- I3 :
-
Intelligent Intraocular Implant
- Lipo-CHT-PCL NS:
-
Lipoidal-chitosan-poly(ε-caprolactone) nanosystem
- LPS:
-
Lipopolysaccharide
- MMER:
-
Molecular Mechanics Energy Relationships
- NS:
-
Nanosystem/s
- OH:
-
Hydroxyl radicals
- PAA:
-
Poly(acrylic acid)
- PCL:
-
Poly(ε-caprolactone)
- SRHS:
-
Stimulus-responsive hydrogel system
- SVH:
-
Simulated vitreous humor
REFERENCES
Herrero-Vanrell R, Refojo MF. Biodegradable microspheres for vitreoretinal drug delivery. Adv Drug Deliv Rev. 2001;52(1):5–16.
Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems: a shift to the posterior segment. Drug Discov Today. 2008;13(3–4):135–43.
Alvarez-Lorenzo C, Concheiro A. Molecularly imprinted polymers for drug delivery. J Chromatogr B. 2004;804(1):231–45.
Barbu E, Verestiuc L, Nevell TG, Tsibouklis J. Polymeric materials for ophthalmic drug delivery: trends and perspectives. J Mater Chem. 2006;16:3439–43.
Panyam J. Inflammation-responsive drug delivery system. Pharmaceutical Sciences, WSU__.htm. Available from: http://research.wayne.edu/idre/db/?view=person&id=42. Relocated in part to: http://research.wayne.edu/idre/tools/faculty-interests.php?id=70.
Ahn BJ, Moshfeghi AA. Implantable Posterior Segment Drug Delivery Devices. Ophthalmology Web; 2008. Available from: http://www.ophthalmologyweb.com/Spotlight.aspx?spid=23&aid=253&headerid=23.
Thilek Kumar M, Pandit JK, Balasubramaniam J. Novel therapeutic approaches for uveitis and retinitis. J Pharm Pharm Sci. 2001;4(3):248–54.
Allergan Inc. EP1750688—Steroid intraocular implants having an extended sustained release for a period of greater than 2 months; 2007. Available from: https://register.epo.org/espacenet/application?number=EP05744945.
Saliba JB, Gomes Faraco AA, Yoshida MI, de Vasconcelos WL, da Silva-Cunha A, Mansur HS. Development and characterization of an intraocular biodegradable polymer system containing cyclosporine A for the treatment of posterior uveitis. Mat Res. 2008;11(2):207–11.
Barcia E, Herrero-Vanrell R, Díez A, Alvarez-Santiago C, López I, Calonge M. Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone. Exp Eye Res. 2009;89:238–45.
Haesslein A, Hacker MC, Ueda H, Ammonb DM, Borazjani RN, Kunzler JF, et al. Matrix modifications modulate ophthalmic drug delivery from photo-cross-linked poly(propylene fumarate)-based networks. J Biomat Sci. 2009;20:49–69.
Ligório Fialho S, Behar-Cohen F, Silva-Cunha A. Dexamethasone-loaded poly(ε-caprolactone) intravitreal implants: a pilot study. Eur J Pharm Biopharm. 2008;68(3):637–46.
Holekamp NM, Thomas MA, Pearson A. The safety profile of long-term, high-dose intraocular corticosteroid delivery. Am J Ophthalmol. 2005;139(3):421–8.
Zeimer R, Goldberg MF. Novel ophthalmic therapeutic modalities based on noninvasive light-targeted drug delivery to the posterior pole of the eye (Drug Delivery to the Posterior Segments of the Eye). Adv Drug Deliv Rev. 2001;52(1):49–61.
Hawkins CL, Davies MJ. Direct detection and identification of radicals generated during the hydroxyl radical-induced degradation of hyaluronic acid and related materials. Free Radical Biol Med. 1996;21:275–90.
Zhao XB, Fraser JE, Alexander C, Lockett C, White BJ. Synthesis and characterisation of novel double crosslinked hyaluronan hydrogel. J Mater Sci Mater Med. 2002;13:11–6.
Saltzman WM. Drug delivery: engineering principles for drug therapy. Oxford: Oxford University Press; 2001.
Hermanson T. Bioconjugate Techniques. 2nd Ed. London: Elsevier; 2008. P. 220.
Choonara YE, Pillay V, Ndesendo VMK, du Toit LC, Kumar P, Khan RA, et al. Design of polymeric nanoparticles by synthetic wet chemical processing strategies for the sustained delivery of anti-tuberculosis drugs. Coll Interf B Biointerface. 2011;87:243–54.
Warhurst DC, Craig JC, Adagu IS, Meyer DJ, Lee SY. The relationship of physico-chemical properties and structure to the differential antiplasmodial activity of the cinchona alkaloids. Malar J. 2003;2:26.
Pearlman DA, Case DA, Caldwell JW, Ross WS, Cheatham TE, De-Bolt III S, et al. AMBER, a package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comp Phys Commun. 1995;91:1–41.
Yu BY, Chung JW, Kwak S-Y. Reduced migration from flexible poly(vinyl chloride) of a plasticizer containing β-cyclodextrin derivative. Environ Sci Technol. 2008;42:7522–7.
Herh P, Tkachuk J, Wu S, Bernzen M, Rudolph B. The rheology of pharmaceutical and cosmetic semisolids. ATS RheoSystems. 1998:12–14.
Takka S. Propranolol hydrochloride—anionic polymer binding interaction. Il Farmaco. 2003;58(10):1051–6.
Nielson LE. Mechanical properties of polymers and composites, vol. 1. New York: Marcel Dekker; 1974.
Yui N, Nihira J, Okano T, Sakurai Y. Inflammation responsive degradation of crosslinked hyaluronic acid gels. J Control Release. 1992;22:105–16.
Tajiri T, Morita S, Sakamoto R, Suzuki M, Yamanashi S, Ozaki Y, et al. Release mechanisms of acetaminophen from polyethylene oxide/polyethylene glycol matrix tablets utilizing magnetic resonance imaging. Int J Pharm. 2010;395(1–2):147–53.
Shaikh RP, Kumar P, Choonara YE, du Toit LC, Pillay. Crosslinked electrospun PVA nanofibrous membranes: elucidation of their physicochemical, physicomechanical and molecular disposition. Biofabr. 2012;4(025002):doi 10.1088/1758-5082/4/2/025002.
Koura Y, Fukushima A, Nishino K, Ishida W, Nakakuki T, Sento M, et al. Inflammatory reaction following cataract surgery and implantation of acrylic intraocular lens in rabbits with endotoxin-induced uveitis. Eye (Lond). 2006;20(5):606–10.
Cheruvu NP, Ayalasomayajula SP, Kompella UB. Retinal delivery of sodium fluorescein, budesonide & celecoxib following subconjunctival injection. Drug Dev Deliv. 2003;3(6):posted on: 3/28/2008.
Kobayashi A, Naito S, Enomoto H, Shiomoi T, Kimura T, Obata K, et al. Serum levels of matrix metalloproteinase 3 (stromelysin 1) for monitoring synovitis in rheumatoid arthritis. Arch Path Lab Med. 2007;131(4):563–70.
Tian Y, Li Y, Xu X, Jin Z, Jiao A, Wang J, et al. A novel size-exclusion high performance liquid chromatography (SE-HPLC) method for measuring degree of amylose retrogradation in rice starch. Food Chem. 2010;118:445–8.
Xie YH, Soh AK. Investigation of non-covalent association of single-walled carbon nanotube with amylose by molecular dynamics simulation. Mat Lett. 2005;59(8–9):971–5.
Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49:1993–2007.
Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem. 2002;277(7):4581–4.
Mlčochová P, Bystrický S, Steiner B, Machová E, Koóš M, Velebný V, et al. Synthesis and characterization of new biodegradable hyaluronan alkyl derivatives. Biopolymers. 2006;82(1):74–9.
Magnani A, Rappuoli R, Lamponi S, Barbucci R. Novel polysaccharide hydrogels: characterization and properties. Polym Adv Tech. 2000;11(8–12):488–95.
Beppu MM, Vieira RS, Aimoli CG, Santana CC. Crosslinking of chitosan membranes using glutaraldehyde: effect on ion permeability and water absorption. J Membr Sci. 2007;301(1–2):126–30.
Kildeeva NR, Perminov PA, Vladimirov LV, Novikov VV, Mikhailov SN. About mechanism of Chitosan cross-linking with glutaraldehyde. Russ J Bioorg Chem. 2009;35:360–9.
Yui N, Nihira J, Okano T, Sakurai Y. Regulated release of drug microspheres from inflammation responsive degradable matrices of crosslinked hyaluronic acid. J Control Release. 1993;25(1–2):133–43.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
(WMV 4278 kb)
Supplementary Table I
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
du Toit, L.C., Carmichael, T., Govender, T. et al. In Vitro, In Vivo, and In Silico Evaluation of the Bioresponsive Behavior of an Intelligent Intraocular Implant. Pharm Res 31, 607–634 (2014). https://doi.org/10.1007/s11095-013-1184-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1184-3