Abstract
Purpose
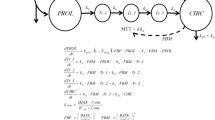
A population pharmacokinetic/pharmacodynamic (PK/PD) model was developed to describe the thrombocytopenia (dose-limiting toxicity) of abexinostat, a new histone deacetylase inhibitor. An optimal administration schedule of the drug was determined using a simulation-based approach.
Methods
Early PK and PK/PD data were analysed using a sequential population modeling approach (NONMEM 7), allowing for the description of a PK profile and platelet-count decrease after abexinostat administration with various administration schedules. Simulations of platelet count with several administration schedules over 3-week treatment cycles (ASC) and over a day (ASD) were computed to define the optimal schedule that limits the depth of thrombocytopenia.
Results
An intermediate PK/PD model accurately described the data. The administration of abexinostat during the first 4 days of each week in a 3-week cycle resulted in fewer adverse events (with no influence of ASD on platelet count profiles), and corresponded to the optimal treatment schedule. This administration schedule was clinically evaluated in a phase I clinical trial and allowed for the definition of a new maximum tolerated dose (MTD), leading to a nearly 30% higher dose-intensity than that of another previously tested schedule. Lastly, a final model was built using all of the available data.
Conclusions
The final model, characterizing the dose-effect and the dose-toxicity relationships, provides a useful modeling tool for clinical drug development.








Similar content being viewed by others
Abbreviations
- ASC:
-
Administration schedule over a cycle (3-week treatment)
- ASD:
-
Administration schedule over a day (e.g. once a day dosing, bid dosing, et cetera)
- BASE:
-
Baseline platelet count (x10^9/L)
- CIRC:
-
Compartment of circulating cells
- EBE:
-
Empirical Bayesian Estimates
- HDACi:
-
Histone deacetylase inhibitor
- kel :
-
Constant rate of elimination
- kprol :
-
Constant rate of proliferation
- ktr :
-
Constant rate between transit compartments
- MTT:
-
Maturation time from PROL to CIRC (h)
- NPDE:
-
Normalized prediction distribution errors
- PK/PD:
-
Pharmacokinetic/Pharmacodynamic
- PROL:
-
Compartment of proliferative cells
- SLOPE:
-
Coefficient of drug decrease (μg/mL)−1
- TRAN:
-
Transit compartment
- VPC:
-
Visual predictive check
- γ:
-
Power factor for the feedback mechanism
References
Batty N, Malouf GG, Issa JP. Histone deacetylase inhibitors as anti-neoplastic agents. Cancer Lett. 2009;280(2):192–200.
Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202.
Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol. 2001;13(6):477–83.
Marks PA, Xu WS. Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem. 2009;107(4):600–8.
Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401(6749):188–93.
Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci U S A. 2004;101(5):1241–6.
Stimson L, Wood V, Khan O, Fotheringham S, La Thangue NB. HDAC inhibitor-based therapies and haematological malignancy. Ann Oncol. 2009;20(8):1293–302.
Bishton MJ, Harrison SJ, Martin BP, McLaughlin N, James C, Josefsson EC, et al. Deciphering the molecular and biologic processes that mediate histone deacetylase inhibitor-induced thrombocytopenia. Blood. 2011;117(13):3658–68.
Giver CR, Jaye DL, Waller EK, Kaufman JL, Lonial S. Rapid recovery from panobinostat (LBH589)-induced thrombocytopenia in mice involves a rebound effect of bone marrow megakaryocytes. Leukemia. 2011;25(2):362–5.
Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clin Cancer Res. 2009;15(12):3958–69.
Rubin EH, Agrawal NG, Friedman EJ, Scott P, Mazina KE, Sun L, et al. A study to determine the effects of food and multiple dosing on the pharmacokinetics of vorinostat given orally to patients with advanced cancer. Clin Cancer Res. 2006;12(23):7039–45.
Matsuoka H, Unami A, Fujimura T, Noto T, Takata Y, Yoshizawa K, et al. Mechanisms of HDAC inhibitor-induced thrombocytopenia. Eur J Pharmacol. 2007;571(2–3):88–96.
Horstmann E, McCabe MS, Grochow L, Yamamoto S, Rubinstein L, Budd T, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352(9):895–904.
Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89(15):1138–47.
Friberg LE, Freijs A, Sandstrom M, Karlsson MO. Semiphysiological model for the time course of leukocytes after varying schedules of 5-fluorouracil in rats. J Pharmacol Exp Ther. 2000;295(2):734–40.
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20(24):4713–21.
Panetta JC, Schaiquevich P, Santana VM, Stewart CF. Using pharmacokinetic and pharmacodynamic modeling and simulation to evaluate importance of schedule in topotecan therapy for pediatric neuroblastoma. Clin Cancer Res. 2008;14(1):318–25.
Schmitt A, Gladieff L, Laffont CM, Evrard A, Boyer JC, Lansiaux A, et al. Factors for hematopoietic toxicity of carboplatin: refining the targeting of carboplatin systemic exposure. J Clin Oncol. 2010;28(30):4568–74.
Soto E, Staab A, Tillmann C, Trommeshauser D, Fritsch H, Munzert G, et al. Semi-mechanistic population pharmacokinetic/pharmacodynamic model for neutropenia following therapy with the Plk-1 inhibitor BI 2536 and its application in clinical development. Cancer Chemother Pharmacol. 2010;66(4):785–95.
Zandvliet AS, Schellens JH, Dittrich C, Wanders J, Beijnen JH, Huitema AD. Population pharmacokinetic and pharmacodynamic analysis to support treatment optimization of combination chemotherapy with indisulam and carboplatin. Br J Clin Pharmacol. 2008;66(4):485–97.
Bender BC, Schaedeli-Stark F, Koch R, Joshi A, Chu YW, Rugo H, et al. A population pharmacokinetic/pharmacodynamic model of thrombocytopenia characterizing the effect of trastuzumab emtansine (T-DM1) on platelet counts in patients with HER2-positive metastatic breast cancer. Cancer Chemother Pharmacol. 2012;70(4):591–601.
Testart-Paillet D, Girard P, You B, Freyer G, Pobel C, Tranchand B. Contribution of modelling chemotherapy-induced hematological toxicity for clinical practice. Crit Rev Oncol Hematol. 2007;63(1):1–11.
Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn. 2003;30(6):387–404.
Lavielle M, Bleakley K. Automatic data binning for improved visual diagnosis of pharmacometric models. J Pharmacokinet Pharmacodyn. 2011;38(6):861–71.
Brendel K, Comets E, Laffont C, Laveille C, Mentre F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res. 2006;23(9):2036–49.
Brendel K, Comets E, Laffont C, Mentre F. Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn. 2010;37(1):49–65.
Quartino AL, Friberg LE, Karlsson MO. A simultaneous analysis of the time-course of leukocytes and neutrophils following docetaxel administration using a semi-mechanistic myelosuppression model. Invest New Drugs. 2012;30(2):833–45.
Sundman-Engberg B, Tidefelt U, Paul C. Toxicity of cytostatic drugs to normal bone marrow cells in vitro. Cancer Chemother Pharmacol. 1998;42(1):17–23.
Zandvliet AS, Karlsson MO, Schellens JH, Copalu W, Beijnen JH, Huitema AD. Two-stage model-based clinical trial design to optimize phase I development of novel anticancer agents. Invest New Drugs. 2010;28(1):61–75.
Calvert AH, Plummer R. The development of phase I cancer trial methodologies: the use of pharmacokinetic and pharmacodynamic end points sets the scene for phase 0 cancer clinical trials. Clin Cancer Res. 2008;14(12):3664–9.
Zohar S, Chevret S. The continual reassessment method: comparison of Bayesian stopping rules for dose-ranging studies. Stat Med. 2001;20(19):2827–43.
Acknowledgments and Disclosures
Quentin Chalret du Rieu and Sylvain Fouliard contributed equally to this work.
The authors would like to thank Pharmacyclics for providing data from the PCYC-401 and PCYC-402 clinical studies.
This work was incorporated into a Ph. D. project (Quentin Chalret du Rieu), granted by Institut de Recherches Internationales Servier.
Quentin Chalret du Rieu, Sylvain Fouliard, Anne Jacquet-Bescond, Renata Robert, Ioana Kloos, Stéphane Depil and Marylore Chenel are employed by Institut de Recherches Internationales Servier. The other authors indicated no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chalret du Rieu, Q., Fouliard, S., Jacquet-Bescond, A. et al. Application of Hematological Toxicity Modeling in Clinical Development of Abexinostat (S-78454, PCI-24781), A New Histone Deacetylase Inhibitor. Pharm Res 30, 2640–2653 (2013). https://doi.org/10.1007/s11095-013-1089-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1089-1