Abstract
Purpose
Drug resistance and severe toxicities are limitations when handling 5-FU. We have developed a triple liposomal formulation of 5-FU combined to 2′-deoxyinosine and folinic acid to improve its efficacy-toxicity balance.
Methods
Stealth liposomes were obtained using the thin-film method. Antiproliferative activity was tested on human colorectal and breast cancer models using sensitive (HT29) and resistant (SW620, LS174t, MDA231) cell lines. In vivo, pharmacokinetics, biodistribution and safety studies were performed in rodents. Finally, efficacy was evaluated using two tumor-bearing mice models (LS174 and MDA231) with response and survival as main endpoints.
Results
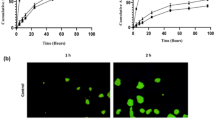
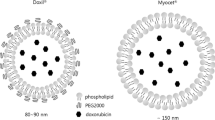
LipoFufol is a 120-nm pegylated liposome, displaying 20–30% encapsulation rates. In vitro, antiproliferative activities were higher than 5-FU, and matched that of FolFox combination in colorectal models, but not in breast. Drug monitoring showed an optimized pharmacokinetics profile with reduced clearance and prolonged half-life. Liposome accumulation in tumors was shown by fluorescence-based biodistribution studies. Beside, milder neutropenia was observed when giving LipoFufol to animals with transient partial DPD-deficiency, as compared with standard 5-FU. In LS174t-bearing mice, higher response and 55% longer survival were achieved with Lipofufol, as compared with 5-FU.
Conclusion
The issues of drug-resistance and drug-related toxicity can be both addressed using a stealth liposomal formulation of modulated 5-FU.









Similar content being viewed by others
References
Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8.
Zhang N, Yin Y, Xu SJ, Chen WS. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13:1551–69.
Yang CG, Ciccolini J, Blesius A, Dahan L, Bagarry-Liegey D, Brunet C, Varoquaux A, Frances N, Marouani H, Giovanni A, Ferri-Dessens RM, Chefrour M, Favre R, Duffaud F, Seitz JF, Zanaret M, Lacarelle B, Mercier C. DPD-based adaptive dosing of 5-FU in patients with head and neck cancer: impact on treatment efficacy and toxicity. Cancer Chemother Pharmacol. 2011;67:49–56.
Zamboni WC. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist. 2008;13:248–60.
Egusquiaguirre SP, Igartua M, Hernández RM, Pedraz JL. Nanoparticle delivery systems for cancer therapy: advances in clinical and preclinical research. Clin Transl Oncol. 2012;14:83–93.
Paolo D. A, liposomal anticancer therapy: pharmacokinetic and clinical aspects. J Chemother. 2004;16:90–3.
Ciccolini J, Peillard L, Evrard A, Cuq P, Aubert C, Pelegrin A, Formento P, Milano G, Catalin J. Enhanced antitumor activity of 5-fluorouracil in combination with 2′-deoxyinosine in human colorectal cell lines and human colon tumor xenografts. Clin Cancer Res. 2000;6:1529–35.
Ciccolini J, Cuq P, Evrard A, Giacometti S, Pelegrin A, Aubert C, Cano JP, Iliadis A. Combination of thymidine phosphorylase gene transfer and deoxyinosine treatment greatly enhances 5-fluorouracil antitumor activity in vitro and in vivo. Mol Cancer Ther. 2001;1:133–9.
Fanciullino R, Giacometti S, Aubert C, Fina F, Martin PM, Piccerelle P, Ciccolini J. Development of stealth liposome formulation of 2′-deoxyinosine as 5-fluorouracil modulator: in vitro and in vivo study. Pharm Res. 2005;22(12):2051–7.
Fanciullino R, Giacometti S, Mercier C, Aubert C, Blanquicett C, Piccerelle P, Ciccolini J. In vitro and reversal of resistance to 5-fluorouracil in colorectal cancer cells with a novel stealth double-liposomal formulation. Br J Cancer. 2007;97:919–26.
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasability of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601.
Waterhouse DN, Madden TD, Cullis PR, Bally MB, Mayer LD, Webb MS. Preparation, characterization, and biological analysis of liposomal formulations of vincristine. Methods Enzymol. 2005;391:40–57.
Blanco E, Hsiao A, Ruiz-Esparza GU, Landry MG, Meric-Bernstam F, Ferrari M. Molecular-targeted nanotherapies in cancer: enabling treatment specificity. Mol Oncol. 2011;5:492–503.
Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–9.
Evrard A, Cuq P, Ciccolini J, Vian L, Cano JP. Increased cytotoxicity and bystander effect of 5-fluorouracil and 5-deoxy-5-fluorouridine in human colorectal cancer cells Transfected with thymidine phosphorylase. Br J Cancer. 1999;80:1726–33.
Petit E, Milano G, Lévi F, Thyss A, Bailleul F, Schneider M. Circadian rhythm-varying plasma concentration of 5-fluorouracil during a 5-day continuous venous infusion at a constant rate in cancer patients. Cancer Res. 1988;48:1676–9.
Etienne-Grimaldi MC, Cardot JM, François E, Renée N, Douillard JY, Gamelin E, Milano G. Chronopharmacokinetics of oral tegafur and uracil in colorectal cancer patients. Clin Pharmacol Ther. 2088;83:413–5.
Bocci G, Di Paolo A, Barbara C, Masi G, Fornaro L, Loupakis F, Allegrini G, Falcone A, Del Tacca M, Danesi R. Pharmacokinetics, a main actor in a many-sided approach to severe 5-FU toxicity prediction. Br J Clin Pharmacol. 2009;67:132–4.
Kovoor PA, Karim SM, Marshall JL. Is levoleucovorin an alternative to racemic leucovorin? a literature review. Clin Colorectal Cancer. 2009;8:200–6.
de Gramont A, Louvet C, André T, Tournigand C, Raymond E, Molitor JL, Krulik M, Modulation of 5-fluorouracil with folinic acid in advanced colorectal cancers. Groupe d'étude et de recherche sur les cancers de l'ovaire et digestifs (GERCOD). Rev Med Interne. 4 (1997) 372 s-378s.
Zamboni WC. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin Cancer Res. 2005;11:8230–4.
Thomas AM, Kapanen AI, Hare JI, Ramsay E, Edwards K, Karlsson G, Bally MB. Development of a liposomal nanoparticle formulation of 5-fluorouracil for parenteral administration: formulation design, pharmacokinetics and efficacy. J Control Release. 2011;150:212–9.
Arias JL, Clares B, Morales ME, Gallardo V, Ruiz MA. Lipid-based drug delivery systems for cancer treatment. Curr Drug Targets. 2011;12:1151–65.
Takeuchi H, Kojima H, Yamamoto H, Kawashima Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J Control Release. 2001;75:83–91.
Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42:463–78.
Milla P, Dosio F, Cattel L. PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Curr Drug Metab. 2012;2012(13):105–19.
Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;16:215–37.
Ciccolini J, Gross E, Dahan L, Lacarelle B, Mercier C. Routine dihydropyrimidine dehydrogenase testing for anticipating 5-fluorouracil-related severe toxicities: hype or hope? Clin Colorectal Cancer. 2010;9:224–8.
Schmoll HJ. Dihydropyrimidine dehydrogenase inhibition as a strategy for the oral administration of 5-fluorouracil: utility in the treatment of advanced colorectal cancer. Anticancer Drugs. 2003;14:695–702.
Fanciullino R, Ciccolini J. Liposome-encapsulated anticancer drugs: still waiting for the magic bullet? Curr Med Chem. 2009;16:4361–71.
Ciccolini J, Mercier C, Evrard A, Dahan L, Boyer JC, Duffaud F, Richard K, Blanquicett C, Milano G, Blesius A, Durand A, Seitz JF, Favre R, Lacarelle B. A rapid and inexpensive method for anticipating severe toxicity to fluorouracil and fluorouracil-based chemotherapy. Ther Drug Monit. 2006;28:678–85.
Meta-analysis Group In Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16:301–8.
Ishikawa T, Sekiguchi F, Fukase Y, Sawada N, Ishitsuka H. Positive correlation between the efficacy of capecitabine and doxifluridine and the ratio of thymidine phosphorylase to dihydropyrimidine dehydrogenase activities in tumors in human cancer xenografts. Cancer Res. 1998;58:685–90.
Acknowledgments
This study was supported by generous grants from the GEFLUC Marseille-Provence and the Association pour la Recherche sur le Cancer (ARC, Grant #1026).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fanciullino, R., Mollard, S., Giacometti, S. et al. In Vitro and In Vivo Evaluation of Lipofufol, a New Triple Stealth Liposomal Formulation of Modulated 5-Fu: Impact on Efficacy and Toxicity. Pharm Res 30, 1281–1290 (2013). https://doi.org/10.1007/s11095-012-0967-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0967-2