ABSTRACT
Purpose
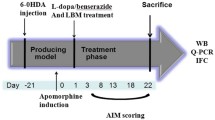
To prepare rotigotine loaded microspheres (RoMS) to achieve continuous dopaminergic stimulation (CDS) for the treatment of Parkinson’s disease (PD) and investigate both the therapeutic benefit and inducibility of AIMs of administration of RoMS combination with L-DOPA in 6-OHDA-leisioned rats.
Methods
Rotigotine was encapsulated into poly(lactic-co-glycolic acid) (PLGA) microspheres by an oil-in-water emulsion solvent evaporation technique. In vitro characteristics and in vivo pharmacokinetics of RoMS either in rat blood or brain (by microdialysis) were investigated. Contraversive rotations and AIMs were observed to investigate the therapeutic benefit and the propensity to induce dyskinesia of RoMS or RoMS combination with L-DOPA in 6-OHDA-lesioned rats.
Results
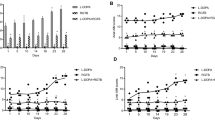
RoMS displayed continuous-release characteristics of rotigotine in animals and exhibited a steady efficacy lasted for 2 weeks in 6-OHDA-lesioned rats. No significant difference of the therapeutic benefit between the treatment of RoMS and pulsatile L-DOPA combination and mono L-DOPA was found. While the dyskinesia was significantly decreased with the treatment of RoMS and pulsatile L-DOPA combination compared to mono L-DOPA.
Conclusions
RoMS could supply an alternative of CDS for the treatment of PD and the study indicates a potential advantage of RoMS for the treatment of mild and advanced PD patient in combination with L-DOPA.






Similar content being viewed by others
Abbreviations
- AIMs:
-
abnormal involuntary movements
- Apo:
-
apomorphine
- CDS:
-
continuous dopaminergic stimulation
- EE:
-
encapsulation efficiency
- im:
-
intramuscular injection
- ip:
-
intraperitoneal injection
- L-DOPA:
-
levodopa
- LID:
-
levodopa induced dyskinesias
- PD:
-
Parkinson’s disease
- PLGA:
-
poly(lactic-co-glycolic acid)
- po:
-
per os
- RoMS:
-
rotigotine loaded microspheres
REFERENCES
David E, Michael P. Parkinson’s disease. http://www.merckmanuals.com/professional/neurologic_disorders/movement_and_cerebellar_disorders/parkinsons_disease.html (accessed 03/05/12)
Braak H, Del TK, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Jenner P. Avoidance of dyskinesia: preclinical evidence for continuous dopaminergic stimulation. Neurology. 2004;62:S47–55.
Rajput AH, Fenton ME, Birdi S, Macaulay R, George D, Rozdilsky B, et al. Clinical-pathological study of levodopa complications. Mov Disord. 2002;17:289–96.
Larramendy C, Taravini IRE, Saborido MD, Ferrario JE, Murer MG, Gershanik OS. Cabergoline and pramipexole fail to modify already established dyskinesias in an animal model of parkinsonism. Behav Brain Res. 2008;194:44–51.
Olanow CW. The scientific basis for the current treatment of Parkinson’s disease. Annu Rev Med. 2004;55:41–60.
Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci. 2001;2(8):577–88.
Nyholm D, Nilsson RAI, Dizdar N, Constantinescu R, Holmberg B, Jansson R, et al. Duodenal levodopa infusion monotherapy vs. oral polypharmacy in advanced Parkinson disease. Neurology. 2005;64:216–23.
Chen L, Togasaki DM, Langston JW, Di Monte DA, Quik M. Enhanced striatal opioid receptor-mediated G-protein activation in L-DOPA treated dyskinetic monkeys. Neuroscience. 2005;132:409–20.
Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med. 2000;342:1484–91.
Nutt JG, Obeso JA, Stocchi F. Continuous dopamine-receptor stimulation in advanced Parkinson’s disease. Trends Neurosci. 2000;23:S109–15.
Olanow CW, Schapira AHV, Rascol O. Continuous dopamine-receptor stimulation in early Parkinson’s disease. Trends Neurosci. 2000;23:S117–26.
Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol. 2006;5:677–87.
Nutt JG. Continuous dopaminergic stimulation: is it the answer to the motor complications of levodopa? Mov Disord. 2007;22:1–9.
Steiger M. Constant dopaminergic stimulation by transdermal delivery of dopaminergic drugs: a new treatment paradigm in Parkinson’s disease. Eur J Neurol. 2008;15:6–15.
Pearce RK, Banerji T, Jenner P, Marsden CD. De novo administration of ropinirole and bromocriptine induces less dyskinesia than L-dopa in the MPTP treated marmoset. Mov Disord. 1998;13:234–41.
Maratos EC, Jackson MJ, Pearce RK, Cannizzaro C, Jenner P. Both short and long-acting D-1/D-2 dopamine agonists induce less dyskinesia than L-DOPA in the MPTP-lesioned common marmoset (Callithrix jacchus). Exp Neurol. 2003;179:90–102.
Bibbiani F, Costantini LC, Patel R, Chase TN. Continuous dopaminergic stimulation reduces risk of motor complications in parkinsonian primates. Exp Neurol. 2005;192:73–8.
Antonini A, Isaias IU, Canesi M, Zibetti M, Mancini F, Manfredi L, et al. Duodenal levodopa infusion for advanced Parkinson’s disease: 12-month treatment outcome. Mov Disord. 2007;22:1145–9.
Katzenschlager R, Hughes A, Evans A, Manson AJ, Hoffman M, Swinn L, et al. Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson’s disease: a prospective study using single-dose challenges. Mov Disord. 2005;20:151–7.
Sujith OK, Lane C. Review: therapeutic options for continuous dopaminergic stimulation in Parkinson’s disease. Ther Adv Neurol Disord. 2009;2:105–13.
Jenner P. A novel dopamine agonist for the transdermal treatment of Parkinson’s disease. Neurology. 2005;65:S3–5.
Rotigotine. http://en.wikipedia.org/wiki/Rotigotine (accessed 03/05/12)
Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease. JAMA. 2000;284:231–8.
Zhang LP. Long acting sustained-release formulation containing dopamine receptor agonist and the preparation method thereof. U.S. Publication 2008/0260846, 2008.
Kehr J, Hu XJ, Yoshitake T, Scheller D. Determination of the dopamine agonist rotigotine in microdialysates from the rat brain by microbore column liquid chromatography with electrochemical detection. J Chromatogr B. 2007;845:109–13.
Przedborski S, Levivier M, Jiang H. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995;67:631–47.
Truong L, Allbutt H, Kassiou M, Henderson JM. Developing a preclinical model of Parkinson’s disease: a study of behaviour in rats with graded 6-OHDA lesions. Behav Brain Res. 2006;169:1–9.
Cannazza G, Di Stefano A, Mosciatti B, Braghiroli D, Baraldi M, Pinnen F, et al. Detection of levodopa, dopamine and its metabolites in rat striatum dialysates following peripheral administration of L-DOPA prodrugs by mean of HPLC-EC. J Pharm Biomed Anal. 2005;36:1079–84.
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioral measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci. 2002;15:120–32.
Stockwell KA, Virley DJ, Perren M, Iravani MM, Jackson MJ, Rose S, et al. Continuous delivery of ropinirole reverses motor deficits without dyskinesia induction in MPTP-treated common marmosets. Exp Neurol. 2008;211:172–9.
Kehr J, Hu XJ, Goiny M, Scheller DK. Continuous delivery of rotigotine decreases extracellular dopamine suggesting continuous receptor stimulation. J Neural Transm. 2007;114:1027–31.
Hammarlund-Udenaes M, Paalzow LK, de Lange EC. Drug equilibration across the blood–brain barrier-pharmacokinetic considerations based on the microdialysis method. Pharm Res. 1997;14:128–34.
Zubair M, Jackson MJ, Tayarani-Binazir K, Stockwell KA, Smith LA, Rose S, et al. The administration of entacapone prevents L-dopa-induced dyskinesia when added to dopamine agonist therapy in MPTP-treated primates. Exp Neurol. 2007;208:177–84.
Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37:111–29.
Schmidt WJ, Lebsanft H, Heindl M, Gerlach M, Gruenblatt E, Riederer P, et al. Continuous versus pulsatile administration of rotigotine in 6-OHDA-lesioned rats: contralateral rotations and abnormal involuntary movements. J Neural Transm. 2008;115:1385–92.
ACKNOWLEDGMENTS & DISCLOSURES
This work was supported by the National Basic Research Program of China (973 Program No. 2012CB724003) and Yantai University (grants YX11Z1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Aiping Wang and Lexi Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, A., Wang, L., Sun, K. et al. Preparation of Rotigotine-Loaded Microspheres and Their Combination Use with L-DOPA to Modify Dyskinesias in 6-OHDA-Lesioned Rats. Pharm Res 29, 2367–2376 (2012). https://doi.org/10.1007/s11095-012-0762-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0762-0